
June 2021
Sampling is a critical factor in the testing of a product. Failure to sample correctly, or to understand the variability associated with sampling, may invalidate the overall test result and lead to an incorrect conclusion.
The main factors to be considered when taking, and/or storing, samples for analysis are:
- correct order of sampling – especially if multiple tests are to be conducted
- ensuring that the sample is representative of the lot
- preventing cross-contamination
- preventing degradation, of the sample and/or the measurand
- the reason for the test, e.g. ongoing quality control, compliance with Regulations or product investigations.
- any statistical analyses that may be required.
Examples of these factors and initial considerations are given below:
Samples for:
- microbial testing should always be taken first, using an aseptic process (see the IFST Handbook of Microbiological Criteria for Foods, 2nd edition)
- physical contamination is next in priority (see separate guidance notes)
- chemical testing samples should be taken last.
Samples must be stored:
- in a suitable container, which may be security tagged/sealed. Essential for legal samples
- at a suitable temperature e.g. samples taken for testing of volatile chemicals, or unsaturated fatty acids, may benefit from frozen storage, but conversely freezing may affect the outcome of microbial testing. In general, samples for microbiology testing should be kept in the state in which they are intended to be stored, i.e. chilled should stay chilled, ambient stay at ambient temperature. N.B. Do not freeze the sample if testing for undeclared freezing. See IFST Information Statement ‘Microbiological Analysis - key considerations’
- under conditions that prevent degradation of the sample, or the analyte to be tested. For example, many vitamins are light sensitive, so samples sent for analysis should be protected from light using dark packaging, or by wrapping the sample packaging in aluminium foil.
- in containers that prevent cross-contamination, e.g. if testing for:
- metal elements, it would not be sensible to wrap samples in aluminium foil
- plasticisers, samples should not be placed into plastic bottles
- glass contamination, the sample must not be placed inside a glass container.
- for a suitable length of time. Samples kept for a long time, before testing, may not be representative of the original product that was sampled.
Sample shipping and storage requirements should also be discussed and agreed with the receiving laboratory.
The overall validity and repeatability of the analytical result is totally dependent upon the sampling protocol employed.
If the product is relatively homogenous, e.g. wines, oils, milk and other liquids, it is often acceptable to sample from any point in the chain, such as from the bulk tank, bottling line or from the finished product in the bottle or carton. However, it should be noted that some bulk tanks will contain sediments, or the liquid may stratify under certain conditions. For example, at low storage temperatures, fats may settle into different layers depending upon the mix of saturated and unsaturated fats present in the bulk tank. These types of products should be agitated and well mixed before sub-samples are taken for testing. Consideration should also be given to any other factors that may affect the reliability of the sampling process e.g. pipework dead ends can be a source of residual contamination.
If the product is more granular (e.g. cereals, figs, nuts, apples), sufficient samples should be taken so that, as far as possible, they are representative of the lot. Complex samples, such as ready meals and muesli, will require great attention to detail if a reliable sample is to be obtained for chemical analysis.
Complex samples for chemical analysis must therefore be blended or combined to produce a homogenous sample before testing. The level of homogeneity to be achieved will be dependent upon:
- the chemical to be tested and the likely source of any contamination - for example, for some naturally occurring chemicals, such as mycotoxins, contamination is often very heterogeneous within a lot. This means that much of the product may contain little or no mycotoxin contamination, but there could be hot spots of high-level contamination[1]. Another example of potential heterogeneity of chemical residues is pesticides on fruit and vegetables[2],.
- unit size - for products with small particle sizes, e.g. blended herbs and spices, and sugar, the distribution of any chemical residue is likely to be fairly consistent. Larger size products, such as unmilled grain, fruit and nuts, should be milled to a small particle size and mixed well before analysis.
- samples size employed by the laboratory - due to increasing sensitivity of testing, many laboratories use less than 2 grams of sample per test. In fact, many laboratories have miniaturised their analytical processes so that only 0.1 to 0.2 gram is used. This means that the small sample to be tested has to be milled, and well mixed, if the final result is to be representative of a lot.
[2] Sampling and Sample Processing in Pesticide Residue Analysis. Agric. Food Chem. 2015, 63, 18, 4395–4404, https://doi.org/10.1021/jf5056985
IFST has obtained permission to use this diagram.
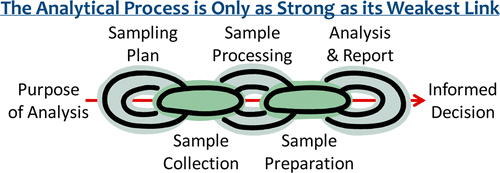
In chemical analyses, subject to the stability of the measurand, it is therefore often advisable to blend a ‘large sample’ and then mix well before taking a representative sub-sample for laboratory testing. Laboratories are a good source of information on correct processing techniques.
Preparation of samples can be done either at source, e.g. in the factory laboratory, or at the testing laboratory itself. Whilst many chemicals are stable during blending and mixing, there are a number, e.g. pesticides and vitamins, that could degrade due to factors such as:
- heat generated during milling to small particle sizes
- the release of enzymes naturally occurring in the food.
In cases of chemical instability, it is often more appropriate for the “granular sample” to be milled or blended at the testing laboratory[3]. It is therefore advisable that the company, or organisation, submitting samples for testing should have a good knowledge of the laboratory protocol, for handling and processing of samples prior to analysis.
Care should therefore be taken to design a complete sample handling process that prevents losses of target chemicals, due to degradation during sample taking, shipment and preparation. In any case the process to be used should be considered and documented.
If efficient and controlled mixing and blending of samples is not possible, for Quality Assurance (QA) purposes, it may be acceptable to use a less homogenous test sample and to conduct analysis on multiple samples, taken randomly from an individual lot. The combined data can then be used to provide an estimate of any product contamination. On-going analysis from QA testing of multiple production batches (plotted versus time) can also be useful. In this approach the more rigorous sampling/mixing/blending procedures would be limited to products that require further investigation, e.g. in the case of potential non-compliance with legal maximum limits.
Another tool for reducing the cost of testing is to employ composite samples. These are often made up from a number of replicate samples (typically n >5), taken across production batches, which are then mixed, or blended, before sending to the laboratory for testing. When using this approach, it is recommended that portions of the sub-samples, used to make the blend, are retained. This allows for the individual samples, used to prepare the composite, to be tested at a later date. This is particularly important if the source of contamination is likely to be heterogenous, e.g. mycotoxins, or when it will be necessary to identify which of the sub-samples may have caused an issue.
The sampling point in the supply chain should also be considered. Whilst it is good practice to test final products, it is also advisable to consider risks associated with inbound goods and raw materials. In some cases, it may be acceptable to rely on certificates of analysis (C of A), provided by suppliers, but depending upon the risk of microbiological, chemical and physical contamination, this should be supplemented by sampling and testing of inbound goods. This may involve a combination of physical checks, rapid ‘in-house’ analyses, e.g. near-infrared, or representative samples being sent to an external laboratory. When comparing laboratory data provided by a supplier with those conducted locally, due consideration should be given to both the sampling protocol and the method of analysis being used at each location. Differences in these protocols or methods, across the world, may have a significant impact on final data interpretation.
The UK Food Standards Agency (FSA) provide guidance on sampling of imported food by Enforcement Officers[4]. Their website is open access and relevant to businesses wishing to understand how samples of imported food are taken for official control purposes.
Institute of Food Science & Technology has authorised the publication of the following Information Statement on Sampling for Food Analysis - key considerations.
This updated Information Statement has been prepared by Matthew Sharman FIFST, peer-reviewed by professional members of IFST and approved by the IFST Scientific Committee.
This information statement is dated June 2021.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
