April 2017
Cyclospora cayetanensis is an emerging infectious disease agent that causes a prolonged and severe diarrhoeal illness known as cyclosporiasis. This infection was first reported in 1979 in Papua New Guinea where an oocyst-like body was found in 3 patients with intestinal infections. It emerged in North America in 1995 and again in 1996 when it was the cause of over two thousand cases of foodborne disease, with no reported deaths. Further outbreaks that occurred in the USA and Canada in 1997–2000 were linked to consumption of the fresh spring crop of raspberries from Guatemala. An outbreak in the US and Canada in 2016 was attributed to fresh produce, although the source of the outbreak was not identified, Cyclosporiasis is endemic to many tropical regions of the world and has been reported in 27 countries; in the UK almost all cases are confined to travelers returning from tropical countries. The microbiological safety of fresh fruit and salad vegetables depends on the avoidance of contamination with pathogenic microorganisms at all stages of production, most particularly in the field. The increasingly global supply chain and increase in consumption of fresh fruits and vegetables has the potential to provide a greater risk of contamination by foodborne pathogens, including Cyclospora.
Cyclospora cayetanensis causes significant morbidity and mortality in sub-tropical and tropical regions including South America and India. The organism has been more frequently reported since the mid-1980s, and it jumped to prominence as an important foodborne human pathogen in May 1996 when 1,465 cases of cyclosporiasis were reported over a few weeks in the United States and Canada(1). The first Cyclospora infection in humans in the USA was diagnosed in 1977 and sporadic outbreaks were reported between 1977 and 1996(2). These were almost exclusively associated with travellers returning from tropical countries; four or five cases were reported in New York annually. In the UK, Cyclospora was first encountered in 1986 in a patient returning from Pakistan (2); 43 cases were reported in 1994, 40 of these were returning travelers. In 2016 high number of Cyclospora cases in the UK were reported (over 320), with the majority being attributed to travel in Mexico.
C. cayetanensis are obligate intracellular parasites of the human small intestine. Oocysts of C. cayetanensis are spherical and 8–10µm in diameter. The oocysts have a 50 nm-thick wall and a bilayer threadlike outer coat(3). Infection rates in the general population of up to 41.6% have been reported(20)
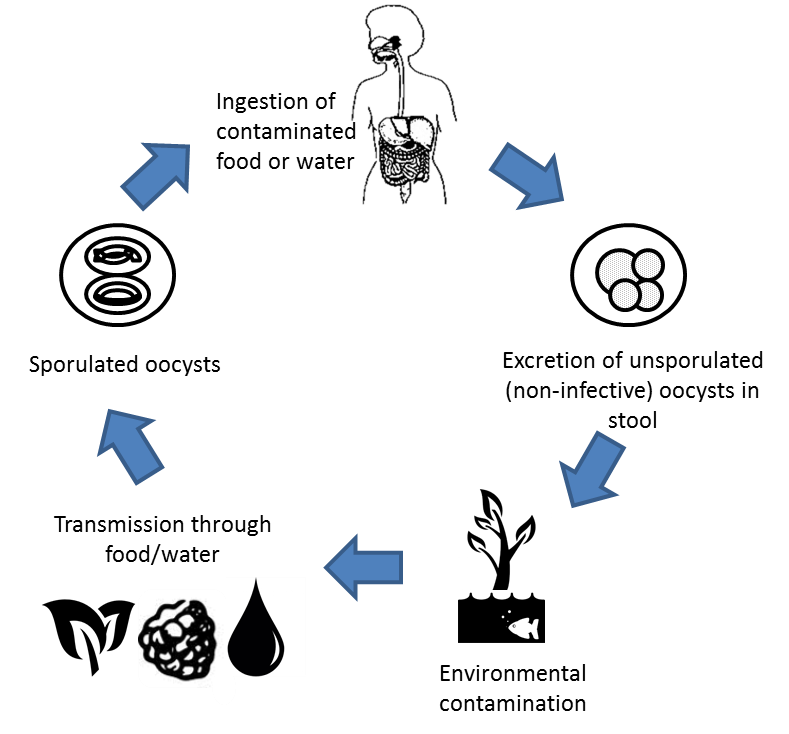
 The oocyst is an undifferentiated sphere containing a morula when it is first shed in the faeces, which is not infective (meaning the direct faecal–oral route utilised by Cryptosporidium does not occur). It may take days to weeks (5–12 days at 26–30°C) for sporulation of the oocysts to occur, depending on the environmental conditions, when the sporont divides into two sporocysts (4 by 6.3 μm) and stieda and substieda bodies. Each sporocyst contains two elongated 1.2 by 9 μm sporozoites that are folded in two with a membrane-bound nucleus and micronemes. When the sporulated oocysts of C. cayetanensis are ingested, the oocyst excsyts, freeing the sporozoites, typically in the jejunum of the small intestine. Once they invade the intestinal epithelial cells, the schizogony process (asexual multiplication and sexual development) starts, where a trophozoite forms that grows into a mature schizont. This first generation contains 8–12 merozoites (asexual form) which are then released, likely through cell rupture, to invade other epithelial cells where the process replicates.
The oocyst is an undifferentiated sphere containing a morula when it is first shed in the faeces, which is not infective (meaning the direct faecal–oral route utilised by Cryptosporidium does not occur). It may take days to weeks (5–12 days at 26–30°C) for sporulation of the oocysts to occur, depending on the environmental conditions, when the sporont divides into two sporocysts (4 by 6.3 μm) and stieda and substieda bodies. Each sporocyst contains two elongated 1.2 by 9 μm sporozoites that are folded in two with a membrane-bound nucleus and micronemes. When the sporulated oocysts of C. cayetanensis are ingested, the oocyst excsyts, freeing the sporozoites, typically in the jejunum of the small intestine. Once they invade the intestinal epithelial cells, the schizogony process (asexual multiplication and sexual development) starts, where a trophozoite forms that grows into a mature schizont. This first generation contains 8–12 merozoites (asexual form) which are then released, likely through cell rupture, to invade other epithelial cells where the process replicates.
After several cycles of the schizogony process, type II meronts (sexual forms) develop, with each cell containing four merozoites. Some of these then form single macrogametes while others divide multiple times to form microgametes. A microgamete fertilizes a macrogamete upon being released, which develops into a zygote. It is the zygote which develops into an oocyst with an environmentally resistant wall. The oocyst passes into the environment in the faeces, as a non-sporulated and immature (non-infectious) oocyst, where it may survive for extended periods in the environment, given the marked seasonality of infection in areas where the disease is endemic(1).
Once in the environment, the oocyst sporulates, due to higher concentrations of atmospheric oxygen, and becomes infectious for humans. Prior to sporulation, the oocyst is non-infective, which is why direct human-to-human transmission does not occur. Evidence suggests that oocysts are continuously excreted during infection, with contamination of food or drinking water leading to human ingestion and infection. The infectious dose has not been determined, but based on outbreak investigations and extrapolations from other coccidians is thought to be relatively low(4), possibly as low as between 10 and 100 oocysts(7).
Life cycle of Cyclospora
There are 16 known Cyclospora species that infect primates, other mammals and reptiles, none of which infect humans. Infection by C. cayetanensis only occurs in humans and there is no known animal reservoir, although the role of animals is of increasing concern. Cyclospora has been identified in source waters in various countries including Nepal and Guatemala(9), wastewater in Tunisia(8) and sewage.
Cyclospora is resistant to pesticides, disinfectants and sanitisers(11) including chlorine and iodine. Part of the reason Cyclospora may be so resistant to disinfectants commonly used in the food industry is their strong adherence to the surfaces of fruits and vegetables. The oocysts do not withstand drying or freezing. It is likely that the organism is inactivated by cooking processes which raise the product temperatures to 70oC, such as pasteurisation(17).
Since the oocysts are resistant to disinfection and are not inactivated by chlorination practices, control measures within a water safety plan for drinking water to manage the potential risk include prevention of source water contamination by human waste, followed by adequate treatment and protection of water during distribution. The resistance of oocysts to disinfectants means that E. coli or thermotolerant coliforms cannot be relied upon as an index of the presence/absence of Cyclospora in drinkingwater supplies(10). Total removal cannot be guaranteed by the use of coagulants, rapid filtration or chemical disinfection with chlorine.
Studies have reported the presence of Cyclospora oocysts in the faeces of chickens, ducks and dogs, however, whether these animals are a natural reservoir host for C. cayetanensis remains to be determined. Shellfish have been proposed to concentrate oocysts from contaminated waters, and a study in 2017 identified wild bivalve mollusks from the Tunisian coast as being contaminated with Cyclospora cayatensis (21)Controlled laboratory studies with freshwater clams (Corbicula fluminea) showed that 48 to 100% of the clams retained Cyclospora oocysts for up to 13 days(4).
Molecular methods including molecular markers such as intervening transcribed spacer regions can identify different genotypes of C. cayetanensis. DNA extraction followed by PCR amplification, PCR sequencing and computer database homology comparison can also be used. Restriction fragment length polymorphism analysis of amplicons can be used to determine genus, species and strain(13).
Stool samples may be sent for microscopy. Up to three samples may be necessary due to the intermittent excretion of this parasite(19). Fluorescent microscopy is a rapid, sensitive, and inexpensive method of diagnosis as Cyclospora oocysts autofluoresce). Trichrome stain and optical brighteners are used to detect spores in faeces, urines, respiratory secretions and other aspirates. Electron microscopy remains an important diagnostic method but its sensitivity is relatively poor. Haematoxylin stain alone for 15 minutes on biopsy specimens by light rather than electron microscopy can detect tissue stages of Cyclospora (15).
Assessment of six different procedures that included Giemsa, trichrome, chromotrope, Gram-chromotrope, acid-fast and safranin stains indicated that heating of faecal smears prior to safranin-based staining yielded a uniform, fast, reliable and easy-to-perform procedure that was superior to acid-fast staining; however the modified Ziehl Neelsen technique produced a variable stain of Cyclospora oocysts. The small size, intracellular nature and poor staining properties with many histological stains contributes to the under-reporting of Cyclospora infections.
Symptoms of cyclosporiasis may include watery diarrhoea which may be explosive, anorexia, weight loss, fatigue, abdominal bloating, nausea and vomiting, low-grade fever and flatulence.
In non-immune individuals, the incubation period is usually 114 days after infection, may be proceeded by a flu-type illness and is typically self-limiting. Acute symptoms may subside and then recur in a waxing–waning pattern or a patient may experience persistent symptoms. The illness usually lasts 6–7 weeks but has been reported to persist for several months. The duration can be several months to a year in patients with HIV, with patients not taking prophylactic trimethoprim/sulfamethoxazole being at risk for developing chronic and debilitating diarrhoea. The disease can be more severe in travellers and expatriates. In countries where Cyclospora is endemic adults are often infected with about 70% of children having asymptomatic infections. Infants are at risk for critical dehydration due to protracted diarrhoea which may result in death. A protracted course of several weeks to months with diarrhoea, dehydration, and weight loss can produce significant morbidity.
Guillain-Barré syndrome and Reiter syndrome (characterised by the triad of ocular inflammation (conjunctivitis, iritis, episcleritis), inflammatory oligoarthritis, and sterile urethritis) have been reported as complications of Cyclospora infection(15).
Cyclosporiasis has been demonstrated to be seasonal in Peru (December through May), Haiti (January through March or April), Guatemala (May through August) and Nepal (May through August), often disappearing for months at a time(6). In the UK the prevalence of C. cayetanensis remains very low. Cases of cyclosporiasis may be misdiagnosed or even missed due to the lack of understanding of the epidemiological and laboratory features of this pathogen(12).
In patients with Cyclospora infection moderate to severe erythema of the distal duodenum is observed, including acute and chronic inflammation, reactive hyperaemia with vascular dilatation and villous capillary congestion, parasitophorous vacuoles (asexual and sexual forms), crypt hyperplasia, epithelial disarray, and partial villous atrophy. Intestinal barrier disruption and malabsorption have been documented during studies.
- In 1990 in a Chicago (US) hospital’s physicians' dormitory, 10 cases were attributed to an infected water source.
- In 1994 in a waterborne outbreak in Nepal, 12 out of a group of British expatriates became ill after drinking out of the military water supply. The mix of river and municipal water had been chlorinated and pumped into tanks beside the houses. Cyclospora oocysts were found in the chlorinated tanks.
Foodborne illness outbreaks have been linked to a variety of fresh and pre-packed produce which is imported, including basil, cilantro, berries, pre-packed salad mix and snap peas.
- In the United States, foodborne outbreaks since the mid-1990s have been linked to fresh produce, including raspberries, basil, snow peas and mesclun lettuce:
- 850 laboratory confirmed cases in May and June 1996, multistate outbreak in the United States and Canada; 20 states, Washington, D.C., and two provinces reported 1465 occurrences. The raspberries attributed as being the food vehicle either definitely came from Guatemala (eight events) or could have come from Guatemala (23 events).
- 1012 cases in 13 U.S. states, the District of Columbia, and one Canadian province.
- In 2000 a Pennsylvanian outbreak (54 cases) was associated with raspberries on a wedding cake; this was unusual in that the raspberries had been frozen.
- 34 cases in Germany in 2000 at a restaurant attributed to one or more of the lettuce varieties or the fresh green leafy herbs (dill, chives, parsley and green onions) used to spice both salads that were provided for lunch.
- In 2001, an outbreak was documented in British Columbia, Canada, with 17 cases of cyclosporiasis associates with Thai basil (imported from the United States).
- 40 laboratory confirmed cases in July 2004; snow peas imported from Guatemala associated with a Cyclospora outbreak in the United States. The snow peas were used to prepare pasta salad.
- 250 cases in Quebec, Canada in 2005. Contaminated fresh basil originating from a Mexican farm, used to prepare an uncooked appetizer, was identified as the source.
- In 2005, in a village close to Ismir, Turkey, 30 cases of abdominal pain, diarrhoea and nausea were reported for school-age children.
- 160 passengers and crew contracted C. cayetanensis on a cruise ship in April 2009, attributed to fresh fruits or vegetables originating in South America.
- 11 people in Florida, United States, in an outbreak following consumption of onions and cilantro in a restaurant in 2011.
- 87 people in Canada in 4 provinces contracted Cyclospora in 2016; the source was not identified.
Agriculture
Farm management practices to reduce the occurrence of Cyclospora:
- Procedures associated with primary production should be conducted under good hygienic conditions and should minimize potential hazards to health due to the contamination of fresh fruits and vegetables.(17)
- A risk management approach (e.g. HACCP) should be utilised, with Cyclospora included in the assessment when assessing the risk and requirement/decision on the technologies to be used.
- Identify the source and distribution of water used and assess the risks for the water being contaminated with protozoa. Sources of water contamination should be assessed and controlled to the extent feasible.
- Follow good agricultural practices that minimize the potential for contaminated water to contact the edible portion of the crop e.g. during irrigation.
- Use appropriate wash methods.
- Vigorous washing of produce not subject to bruising or injury may increase the likelihood of pathogen removal with brush washing being more effective than washing without brushes.
- For some types of produce (apples, celery, tomatoes) the temperature of wash water should be greater than that of the produce or the resultant pressure differential may cause water to be pulled into the plant material. Pathogens present on the produce surface or in the water may become internalized therefore temperature of the wash water must be considered for certain types of produce.
- Optimise management and storage of manure and slurry to reduce the infectivity of oocysts through raised temperature and ammonia levels.
- Reduce run off from animal farms into drinking water supplies, for example by planting strips of grass or vegetation. This may help to trap sediment and therefore reduce the organic matter contamination.
- Use potable water for making up insecticides and pesticides.
Processing including water suppliers, industries or sectors that use fresh product and operations in which contaminated process or waste water could be used:
- All water that is to be used in direct contact with food and food contact surfaces must be of potable quality and should be free of pathogenic microorganisms.
- A consistent supply of food-safe water for use in direct contact with foods and food contact surfaces is required.
- When water to be used for drinking, bottling or vending can be treated prior to use, microfiltration membranes or similar can be incorporated in conjunction with other measures (such as UV treatment, reverse osmosis or pasteurisation/distillation) to reduce further the likelihood of Cyclospora contamination.
- Water to be used as an ingredient (juices, beer and so on) or in the making of baby formula should be filtered and /or other treatment systems used.
- A risk management approach (e.g. HACCP) should be utilised and Cyclospora included in the assessment when assessing the risk and requirement/decision on the technologies to be used, taking into account:
- Water source
- pH (naturally acidity, carbonation)
- Temperature of product
- Presence of preservatives
- Final consumer
- Whether chemical technologies are sufficient or physical removal methods are required
Recommendations for food handlers with Cyclospora infection
Recommendations for food handlers with diarrhoeal illness:
- Exclude food handlers for 48 hours after the first normal stool.
- Refer for appropriate diagnostic testing
Recommendations for food handlers with diarrhoeal illness and laboratory confirmed Cyclospora infection:
- Exclude from work until diarrhoea resolves;
- Refer for antimicrobial therapy :
- Per routine, re-emphasize the importance of proper hand washing before food preparation and after using the toilet; and
- Check local regulations to see if other measures are required.
For further information see the Food Standards Agency guidance ‘Fitness to Work - Regulatory guidance and best practice advice for Food Business Operators (2009)’ (http://www.food.gov.uk/multimedia/pdfs/publication/fitnesstoworkguide09v3.pdf [1])
- Strausbaugh [2], L.J. & Herwaldt [3], B.L. (2000) Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis, 31 (4), 1040–1057.
- Bendall, R.P. & Chiodini, P.L. (1995). The epidemiology of human Cyclospora infection in the UK. In: Betts, W.B., Casemore, D., Fricker, C. & Watkins, J. (eds), Protozoan Parasites and Water. The Royal Society of Chemistry, Cambridge University Press.
- Dixon [4], B.R., Bussey [5], J.M., Parrington [6] L.J. & Parenteau [7] M. (2005) Detection of Cyclospora cayetanensis Oocysts in Human Fecal Specimens by Flow Cytometry. J Clin Microbiol, 43(5), 2375–2379.
- Ortega [8], Y.R. & Sanchez [9]doi, R. (2001) Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin Microbiol Rev, 23 (1), 218–234.
- http://www.cdc.gov/parasites/cyclosporiasis/ [10]
- http://emedicine.medscape.com/article/236105-overview [11]
- http://www.nbbcfood.info/foodmatters/foodsafetyatoz/foodsafetydetails.as...
- Ben Ayed L., Yang W., Widmer G., Cama V., Ortega Y., Xiao L. (2012) Survey and genetic characterization of wastewater in Tunisia for Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi, Cyclospora cayetanensis and Eimeria spp. J Water Health, 10(3), 431–444.
- Dowd S.E [13]., John D., Eliopolus J., et al. (2003) Confirmed detection of Cyclospora cayetanesis, Encephalitozoon intestinalis and Cryptosporidium parvum in water used for drinking. J Water Health, [14] 1(3),117–123.
- World Health Organisation. Guidelines for drinking-water quality: Vol.1, Recommendations. – 3rd ed. World Health Organization, Rome. Available from: URL: http://www.who.int/entity/water_sanitation_health/dwq/fulltext.pdf [15]
- Ortega-Pierres, M.G., Cacciņ, S., Fayer, R., Mank, T. & Smith, H. (eds) (2009) Giardia and Cryptosporidium. CABI.
- Sajjad Raja, N. & Schelenz [16], S. (2010) Cyclospora cayetanensis causing diarrhoea in adults in Norfolk, England: report of two cases and review of literature. Scott Med J, 55, 58.
- Curry, A. & Smith, H.V. (1991) Emerging pathogens and microsporidia. Parasitology, 117 (7), 143–159.
- Dixon, B.R., Parrington, L., Cook, A., Pollari, F. & Farber, J. (2013) Detection of Cyclospora, Cryptosporidium, and Giardia in ready-to-eat packaged leafy greens in Ontario, Canada. J Food Prot [17], 76 (2), 307–313.
- Quintero-Betancourt, W [18]., Peele, E.R [19]. & Rose, J.B [20]. (2002) Cryptosporidium parvum and Cyclospora cayetane: a review of laboratory methods for detection of these waterborne parasites. J Microbiol Methods [21], 49(3), 209–224.
- Connor,B.A., Johnson, E.J. & Soave, R. (2001) Reiter syndrome following protracted symptoms of Cyclospora infection. [22] Emerg Infect Dis, 7(3), 453–454.
- Lawley, R., Curtis, L. & Davis, J. (2008) Food Safety Hazard Guidebook [23]. RSC Publishing, London.
- Codex Alimentarius (2010) Code of hygienic practice for fresh fruits and vegetables, 2010 ref CAC/RCP 53-2003. Available from: URL: http://www.codexalimentarius.org/standards/list-of-standards/en/ [24]
- Health Protection Agency. Public Health England Parasitology Reference Laboratory, Department of Clinical Parasitology, Hospital for Tropical Diseases. Available from: URL: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1316424721258 [25]
- Chacín-Bonilla L, Barrios F. (2011) Cyclospora cayetanensis: biology, environmental distribution and transfer. Biomedica. 2011 Mar;31(1):132-44
- Ghozzi K, Marangi M, Papini R, Lahmar I, Challouf R, Houas N, Ben Dhiab R, Normanno G, Babba H, Giangaspero A. . First report of Tunisian coastal water contamination by protozoan parasites using mollusk bivalves as biological indicators. Mar Pollut Bull. 2017 Apr 15;117(1-2):197-202.
This updated Information Statement has been prepared by Julie Ashmore CSci FIFST, in cooperation with IFST’s Scientific Committee.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
