
This updated Information Statement, dated March 2014, prepared by Prof J Ralph Blanchfield in cooperation with the IFST Scientific Committee, replaces the version published in November 2011
The term "phytosterols" covers plant sterols and plant stanols. Plant sterols are naturally occurring substances present in the diet principally as minor components of vegetable oils. Plant stanols, occurring in nature at a lower level, are hydrogenation compounds of the respective plant sterols. An elevated level of blood cholesterol is one of the well established risk factors for coronary heart disease; both plant sterols and plant stanols are effective in lowering plasma total and low density lipoprotein (LDL) cholesterol which occurs by inhibiting the absorption of cholesterol from the small intestine. In order to achieve a cholesterol lowering benefit approximately 1g/day of plant sterols or plant stanols need to be consumed. In comparison, normal dietary intake (i.e. without fortification) is between 200-400mg/day plant sterols whereas the normal dietary intake of plant stanols is negligible.
The enrichment of foods such as margarines with phytosterol esters has been an important development in functional foods to enhance the cholesterol lowering ability of traditional food products. The consumption of average amounts of spread per day (approximately 20g in Western Europe) supplemented with between 8-10% plant sterol or plant stanol lowers serum total cholesterol and LDL cholesterol by 8-13% - this is equivalent to the consumption of between 1.6 - 2.0g plant sterols or plant stanols per day. Plant sterols and plant stanols appear to be without hazard to health, having been shown to produce no adverse effects in a large number of human studies. They show no evidence of toxicity even at high dose levels and gastro-intestinal absorption is low. In the United States, a panel of independent experts has concluded that vegetable oil plant sterol esters and plant stanol esters are safe for use as an ingredient in vegetable oil spreads. Furthermore in the European Union an opinion from the Scientific Committee on Food (SCF) concluded that the use of phytosterol esters in yellow fat spreads (maximum level of 8% free phytosterols) is safe for human use. More recently the SCF and its successor, the European Food Safety Authority (EFSA) through its Scientific Panel on Dietetic Products, Nutrition and Allergies, have recommended that sterol-containing foodstuffs should not be consumed in amounts resulting in total phytosterol intakes exceeding 3 g/day. However, more recent approval for their incorporation in other foods has made it more difficult for consumers to know whether they are adhering to that advice.
EFSA in 2008 judged that the available scientific evidence justified a health claim “Plant sterols have been shown to lower/reduce blood cholesterol. Blood cholesterol lowering may reduce the risk of coronary heart disease". An EU Regulation requires that on the label “there shall be a statement that the product is not intended for people who do not need to control their blood cholesterol”.
Phytosterols (including plant sterols and stanols) are natural components of edible vegetable oils such as sunflower seed oil and, as such are natural constituents of the human diet. The plasma cholesterol-lowering properties of plant sterols have been known since the 1950s (Pollak, 1953). Early studies examined the cholesterol-reducing potential of plant sterols using up to 25g/day consumed in solid crystalline form. Over the years, however, it was established that sterols dissolved in edible fat products are more effective at reducing blood cholesterol levels than sterols in crystalline form. It is difficult to incorporate free sterols into edible fats/oils because of their insolubility, whereas sterols esterified to fatty acids are more fat soluble. In the intestine, most sterol esters are hydrolysed to free sterols as part of the normal digestive process.
Plant stanols are the hydrogenated counterparts of the plant sterols but are less abundant in nature than the corresponding plant sterols. Consequently, the normal dietary intake of plant stanols is much less than that of plant sterols. An elevated level of blood cholesterol is one of the well established risk factors for coronary heart disease. Blood cholesterol levels can be decreased by following diets which are low in saturated fat, high in polyunsaturated fat and low in cholesterol. Although considerable achievements have been made in terms of knowledge and education, consumers still find it difficult to follow healthy eating advice. The enrichment of foods such as margarines with plant sterols and stanols is one of the recent developments in functional foods to enhance the cholesterol-lowering ability of traditional food products.
Plant sterols have a role in plants similar to that of cholesterol in mammals, e.g. forming cell membrane structures. Plant sterols fall into one of three categories: 4-desmethylsterols (no methyl groups); 4-monomethylsterols (one methyl group) and 4,4-dimethylsterols (two methyl groups). The most common plant sterols are β-sitosterol, campesterol and stigmasterol and structurally these are very similar to cholesterol, belonging to the class of 4-desmethylsterols (see Figure 1).
Figure 1: Structure of common plant sterols, cholesterol and a plant stanol
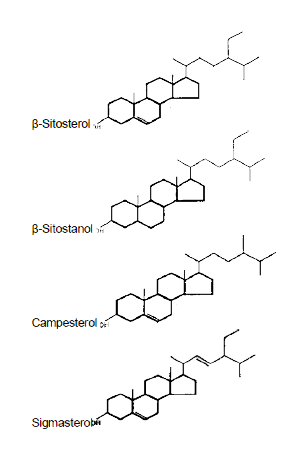
Plant stanols belong to the group of 4-desmethylsterols. Plant stanols are hydrogenation products of the respective plant sterols, e.g. campestanol/campesterol and sitostanol/sitosterol, and are found in nature at very low levels.
When edible oils undergo normal refining, plant sterols are partially extracted together with some tocopherols (in the process of natural vitamin E production). It is estimated that 2500 tonnes of vegetable oil needs to be refined to produce 1 tonne of plant sterols. Plant stanols are obtained by hydrogenation of the plant sterols.
Another source of plant sterols is tall oil, derived from the process of paper production from wood and approximately 2500 tonnes of pine is required to produce 1 tonne of plant sterols. Tall oil also contains a higher proportion of plant stanols (primarily β-sitostanol) than do vegetable oils.
Prior to fortification, average natural background dietary intakes of plant sterols/stanols in 'Western' diets were between 200 - 400mg/day with vegetarians having higher intakes than those on mixed diets. Cooking oils, margarines and peanut butter (containing between 100 and 500mg/100g) have until recently been the main sources of plant sterols in typical Western diets. Legumes (up to 220mg/100g) and some seeds (e.g. sunflower and sesame 500 - 700mg/100g) are also good sources, while other vegetables and fruits contain slightly lower amounts of plant sterols. The intake of plant stanols is normally much lower. In nature, plant sterols can be in the free form or predominantly esterified with long chain fatty acids or with phenolic acids as in rice bran oil (ferulates) and shea butter (cinnamates). In the intestine, most sterol esters are hydrolysed to free sterols as part of the normal digestive process.
It has been known for fifty years that plant sterols can lower blood cholesterol. Plant sterols have been the subject of numerous high dose (up to 25g/day), long term clinical trials to assess their effects on blood cholesterol levels. Over 1800 people in total have participated in these studies, conducted since the early 1950s (Pollak and Kritchevsky, 1981). From the 1950s to the 1980s, a preparation of predominantly β -sitosterol [Cytellin, Eli Lilly] was marketed in the USA to treat hypercholesterolaemia. No adverse effects were reported.
Yellow fat spreads and soft cheese products, supplemented with plant sterols and plant stanols, are now well-established on the market. The consumption of average amounts of spread per day (approximately 20g in Western Europe) supplemented with between 8 - 10% plant sterol lowers serum total cholesterol and LDL cholesterol by 8 - 13% (Weststrate and Meijer,1998; Hendriks et al., 1999). This is equivalent to the consumption of between 1.6 - 2.0g plant sterols per day. In an efficacy study on plant stanols a daily consumption of 24g of spread, containing 2-3g of plant stanol esters, total serum cholesterol and LDL cholesterol were lowered by up to 6.4% and 10.1% respectively (Nguyen et al., 1999). A dose dependent decrease in total and LDL cholesterol was observed with increasing levels of plant stanols esters up to a level of 1.6g/day. However, increasing the dose from 2.4g to 3.2g did not provide any clinically important additional effect (Hallikainen et al., 2000). Plant sterols and plant stanols appear to be equally effective in lowering plasma total and LDL cholesterol (Weststrate and Meijer, 1998; Law, 2000) and in a study with ileostomy subjects cholesterol absorption was found to be inhibited equally by esterified plant sterol and β-sitostanol esters (Normén et al., 2000).
In a clinical trial to examine the effects of plant sterols and plant stanols-enriched margarine on plasma lipid and phytosterol concentrations, de novo cholesterol synthesis and absorption rates in the context of a controlled diet, fifteen hypercholesterolemic men were fed either a diet of prepared foods alone or a diet containing 1.84 g plant sterols/stanols/day for 21 days in a cross-over study design. Dietary effects on total and LDL cholesterol concentrations were observed, although concentrations were lower with the plant sterol/stanol-enriched margarine than with the control diet on day 21 (P < 0.05). LDL-cholesterol concentrations on day 21 had decreased by 3.9% (P < 0.01) and 12.9% 0.001) with the control diet and plant sterol-enriched margarine, respectively. For the plant stanol-enriched margarine, the decrease was 7.9% (p<0.01). High density lipoprotein (HDL)-cholesterol concentration did not change significantly (Jones et al., 2000).
Although people previously consumed plant sterols every day in their normal diet, the amount was not great enough to have a significant blood cholesterol lowering effect. In order to achieve a cholesterol-lowering benefit, approximately 1 g/day of plant sterols need to be consumed (Hendriks et al., 1999).
The consumption of plant sterols and plant stanols lowers blood cholesterol levels by inhibiting the absorption of dietary and endogenously-produced cholesterol from the small intestine and the plant sterols/stanols are only very poorly absorbed themselves. This inhibition is related to the similarity in physico-chemical properties of plant sterols and stanols and cholesterol and it is generally accepted that there are two possible mechanisms by which this inhibition occurs:
The co-precipitation of cholesterol and plant sterols/stanols
In the intestinal lumen, cholesterol is found in solution with other fats. However, as monoglycerides and fatty acids are absorbed from the gut, concentration of the less well absorbed substances, e.g. sterols increases. When their concentration reaches a critical level, similar substances can precipitate from the solution. This may happen with cholesterol and plant sterols/stanols, because of their similarity in structure. Both cholesterol and plant sterols/stanols in the free state are poorly soluble in fat and micelles and in fact mutually limit each other’s solubility. Hence the greater the amount of plant sterols/stanols, the lower the solubility and perhaps the greater the amount of cholesterol precipitated. Cholesterol in the crystalline form cannot be absorbed.
The competition for space in mixed micelles
Micelle or mixed micelles are very efficient detergent-structures that solubilise the lipids secreted in the small intestine. Mixed micelles are composed of bile salts, phospholipids, tri, di- and monoglycerides, fatty acids, free cholesterol and fat soluble micronutrients. As there is only limited capacity in the micelles for carrying cholesterol, compounds with similar structures to cholesterol such as plant sterols and stanols, can compete with cholesterol for this space. Therefore increasing the amounts of plant sterols and stanols results in less and less cholesterol in mixed micelles and thereby decreased absorption of cholesterol from the gut.
In the process of absorption, cholesterol in the micelles is transported from the lumen of the small intestine across the intestinal mucous and into the lymph. The precise mechanism of cholesterol transport from the micelles into the intestinal cells is not fully understood. However, it is known that micelles are not absorbed intact, but that the fats, including cholesterol, pass through the brush border membrane into the cells, probably with the involvement of binding proteins and passive transport mechanisms.
Although people consumed plant sterols every day in their normal diet (i.e. excluding foods fortified with plant sterols), the amount is not great enough to have a significant blood cholesterol lowering effect. In order to achieve a cholesterol-lowering benefit, approximately 1 g/day of plant sterols need to be consumed (Hendriks et al., 1999).
Awad et al (2000a) reported on mice with human cancer tumours fed either a phytosterol diet or a cholesterol diet. Tumour size in animals fed the phytosterols was 33% smaller and had 20% fewer shifts of cancer cells to lymph nodes and lungs than in the cholesterol diet group.
Several theories regarding the mechanism of action of phytosterols as a protective factor include inhibiting of cell division, stimulating death of tumour cells and modifying some of the hormones that are essential to tumour growth [Awad et al (2000b), (2000c) (2003); Li et al (2001)].
A cancer-protective effect has been claimed for phytosterols in humans (Bradford & Awad, 2007; Woyengo T A et al, 2009).
The blood cholesterol-lowering effect of plant sterols has been investigated in a large number of clinical trials on over 1800 people, with up to 25g per day and durations up to three years. No significant adverse effects have been observed in the decades of medically supervised clinical efficacy testing and the general clinical use of plant sterols.
In addition, both plant sterols and plant stanols have been subjected to a thorough toxicological evaluation. Studies on the absorption, distribution, metabolism and excretion have shown that plant sterols are poorly absorbed from the intestine (1 - 10%,) and that portion which is absorbed is rapidly eliminated via excretion in the bile (Sanders et al., 2000).
In a 13 week feeding study of plant sterol esters in rats (Hepburn et al., 1999), no adverse effects were seen at levels up to 3900mg/kg body weight/day (8.1% plant sterol esters in the diet; equivalent to 5.0% free plant sterol), the highest level tested. In a similar study plant stanol esters were reported to have no adverse effects at levels up to 1.78%, equivalent to 1.0% free stanols (Turnbull et al., 1999).
There are some suggestions from the literature that plant sterols are oestrogenic (Mellanen et al., 1996). However, in a series of in vitro assays with vegetable oil plant sterols it has been shown that they do not bind to either the human oestrogen receptor or the immature rat oestrogen receptor. Furthermore, there was no indication of oestrogenicity in an in vivo rat uterotrophic assay (Baker et al., 1999).
Plant stanol esters have similarly been proven not to possess any oestrogenic potential in an in vitro assay (proliferation of MCF-7 cells) or in an in vivo rat uterotrophic assay (Turnbull et al., 1999). The reproductive toxicity of both plant sterols and plant stanols has also been assessed in a two generation reproductive toxicity study in the rat. There were no adverse effects of plant sterols up to approximately 4000mg/kg/day (8.1% vegetable oil sterol esters in the diet; equivalent to 5% free plant sterols); highest level tested (Waalkens-Berendsen et al., 1999). No adverse findings were reported for plant stanol esters up to levels of 4.38% in the diet, equivalent to 2.5% free stanols (Whittaker et al., 1999).
A series of human studies with vegetable oil plant sterol esters in spreads with intakes of up to 8.6g of plant sterols/day for 4 weeks have been conducted. Clinical chemistry, haematology, bacterial profile of the gut microflora and general physical condition were evaluated and no adverse effects were detected (Weststrate and Meijer, 1998; Weststrate et al., 1999s; Hendriks et al., 1999; Ayesh et al., 1999).
A detailed assessment of studies on toxicity, reproductive toxicity, genotoxicity and estrogenicity of phytosterol and phytostanol esters has been carried out by the sixty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (World health Organisation, 2009). The Committee evaluated the toxicological studies with a range of phytosterols, phytostanols and their esters, together with several double-blinded, placebo-controlled human studies, in which these substances were added to the diet. As phytosterol and phytostanol esters and mixtures of phytosterols and phytostanols generally show similar effect profiles, the Committee considered establishing a group acceptable daily intake (ADI). Using the combined evidence from several short-term (90-day) studies of toxicity, the Committee identified an overall NOAEL1 of 4200 mg/kg bw per day. The Committee considered the margin between this overall NOAEL and the lowest LOAEL from the 90-day toxicity studies of 9000 mg/kg bw per day as adequate for this overall NOAEL to be used as the basis for establishing an ADI. This conclusion is supported by the results of the available studies of reproductive toxicity.
The Committee established a group ADI of 0–40 mg/kg bw for the group of phytosterols, phytostanols and their esters, expressed as the sum of phytosterols and phytostanols in their free form, based on the overall NOAEL, to which a safety factor of 100 was applied. This safety factor incorporates a factor of 10 for interspecies differences and a factor of 10 for intraspecies differences. Based on the availability of a range of studies in humans, which includes two 1-year studies, the Committee considered the safety factor of 100 as sufficient to also account for deficiencies in the database, such as the absence of chronic studies in experimental animals. As there is no evidence for genotoxicity of phytosterols or phytostanols and their esters and no indication of potential for carcinogenicity from the available toxicity studies, the Committee did not see a need for a carcinogenicity study to be performed. Based on available data, the Committee concluded that dietary exposure to phytosterols and phytostanols would typically be within the ADI range of 0–40 mg/kg bw.
There is a very rare condition, known as phytosterolaemia (sitosterolaemia), in which sufferers have an inborn error in the metabolism of phytosterols. Only a few dozen cases are known worldwide and, for example, it has been reported that in The Netherlands there is only one known case. Due to the nature of the disease, sufferers are aware of their condition and should avoid additional intake of phytosterols.
In the United States a panel of independent experts concluded that vegetable oil sterol esters meeting appropriate food-grade specifications and produced by current good manufacturing practice (21 C.F.R. § 182.1(b)), are safe for use as an ingredient in vegetable oil spreads in amounts not to exceed 20%. It is the Panel's opinion that qualified experts in the field would generally recognise that vegetable oil sterol esters are safe for this use, i.e. that vegetable oil sterol esters are generally recognised as safe (GRAS). The US Food and Drug Administration (FDA) have also cleared a spread containing up to 20% of plant sterol ester, and one containing plant stanol ester, on the basis of the GRAS recognition.
In Switzerland authorisation for Becel pro.activ, a special spread enriched with plant sterols, was given in September 1999 by the Swiss Health Office (Bundesamt fur Gesundheit; BAG).
In the European Union (EU) plant sterol esters for use in margarines/spreads has been reviewed under the EU Novel Foods Regulation (Regulation (EC) No 25 8/97) and the EU Scientific Committee on Food (SCF) concluded that the use of phytosterol-esters in yellow fat spreads (maximum level of 8% free phytosterols) is safe for human use. In addition, the SCF has expressed a general view on the long-term effects of the intake of elevated levels of phytosterols from multiple dietary sources. The Committee concluded that a numerical upper level for the total daily intake of phytosterols could not be established on the basis of the data available. In consideration of the dosages found to be effective for cholesterol lowering, without evidence of additional benefit at higher intakes and the possibility that high intakes might induce undesirable effects, it was considered prudent to avoid plant sterol intakes exceeding 3 g/day (SCF, 2002a). Furthermore, in the evaluation of the first application concerning plant sterols in yellow fat spreads, the SCF required that the applicant should establish a surveillance program accompanying the marketing of the product to obtain data on consumption and for further investigation of possible health effects, including among others the effects on plasma β-carotene levels. The results of this task have also been assessed by the SCF in its opinion on a report on post-launch monitoring of yellow fat spreads with added phytosterol esters (SCF, 2002b).
However, a yellow fat spread containing plant stanol esters was already legally on the market in the European Union without being subjected to review, because it was marketed in a Member State before the Novel Foods Regulation came into force. A similarly-based cheese spread by the same manufacturer considering it as merely a variant of the yellow fat spread, is also on the market in the UK, but was withdrawn from the shops in The Netherlands on instructions from the Dutch authority which regarded it as a separate novel food requiring prior approval.
The UK Advisory Committee on Novel Foods and Processes (ACNFP), responsible for assessing the safety of these products, asked the then Food Advisory Committee (FAC) to advise on the associated labelling issues. FAC concluded that the products should be clearly labelled so that those with an inborn error in the metabolism of phytosterols (phytosterolaemia) could avoid them. It also agreed that consumers should be informed that phytosterol ester-containing products are not nutritionally appropriate for young children and breast feeding women (as they did not need to reduce their blood cholesterol levels and there was a possibility that the products could affect vitamin A status). It stressed that this did not present a safety hazard and that the advice was purely for nutritional benefit rather than a health warning. Initially it recommended that the most appropriate way to do this would be through information circulated via GPs and other health professionals and through suitable magazines. Subsequently, however, it modified its earlier view and recommended that this information should also be provided on the product labels. This advice was welcomed by the then Committee on Medical Aspects of Food and Nutrition Policy (COMA).
Subsequently the Scientific Panel on Dietetic Products, Nutrition and Allergies of the European Food Safety Authority (EFSA) recommended that sterol-containing foodstuffs should not be consumed in amounts resulting in total phytosterol intakes exceeding 3 g/day. With regard to β-sitostanol and campestanol, higher contents of 35% and 15%, respectively, were accepted by the Panel since these compounds or their esters have been sufficiently tested toxicologically. The Panel also reiterated the recommendations expressed in the previous SCF opinions in relation to the need for risk management measures to minimise the likelihood of a daily intake exceeding 3g phytosterols/phytostanols, the provision of appropriate information to consumers regarding the need for regular consumption of fruits and vegetables to address the potential β-carotene lowering effect of the product, and the particular circumstances of phytosterolaemic patients, people under cholesterol-lowering medication and of women during pregnancy and lactation. However, more recent approval for the incorporation of phytosterols/phytostanols in other foods, including yogurts and fruit juices, has made it more difficult for consumers to know whether they are adhering to that advice. A US snack food manufacturer has reported that its tortilla chips, potato chips and oatmeal squares incorporate phytosterols (Anon, 2011).
In December 2004 the EU Commission published Regulation 608/2004/EC concerning the labelling of foods and food ingredients with added phytosterols, phytosterol esters, phytostanols and/or phytostanol esters, requiring such products to be labelled with additional information including the words “with added plant sterols/plant stanols”.
In July 2013 Commission Regulation (EU) No 718 2013,amending Regulation (EC) 608/2004 concerning the labelling of foods and food ingredients with added phytosterol esters, phytostanols and/or phytostanol esters required in Article 1
“there shall be a statement that the product is not intended for people who do not need to control their blood cholesterol level.”
Anon (2011). Phytosterols, Food Technology, 65 (9), 72 and 74. http://www.foodprocessing.com/articles/2010/wellness_savorysnacks.html or http://corazonas.com/events-and-news/corazonas-foods-launches-first-snac...
American Heart Association (2001). Science Advisory “Stanol/Sterol Ester-Containing Foods and Blood Cholesterol Levels”, Circulation. 2001;103:1177-1179.
http://circ.ahajournals.org/content/103/8/1177.full
Awad A B et al (2000a). Peanuts as a Source of β-sitosterol, a Sterol with Anticancer Properties. Nutrition and Cancer. 36(2),238-41.
Awad A B et al (2000b). Dietary Phytosterol Inhibits the Growth and Metastisis of MDA-MB-231 Human Breast Cancer Cells Grown in SCID Mice. Anticancer Research. 20,821-24.
Awad A B et al (2000c). Phytosterols as Anticancer Dietary Components: Evidence and Mechanism of Action. Journal of Nutrition, 130, 2127-30.
Awad A B et al (2001). European Journal of Cancer Prevention.” In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells”. 10(6), 507-513.
Awad A B et al (2003). Effect of phytosterols on cholesterol metabolism and MAP kinase in MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 14,111-119.
Ayesh R et al (1999). Safety evaluation of phytosterol-esters. Part 5: Faecal-short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidemic volunteers consuming a controlled diet either with or without a phytosterol-ester enriched margarine. Food and Chemical Toxicology, (1999) 37, (12), 1127-1138.
http://www.sciencedirect.com/science/article/pii/S027869159900109X
Baker V A et al (1999). Safety evaluation of phytosterol esters. Part 1: Assessment of oestrogenicity using a combination of in vivo and in vitro assays. Food and Chemical Toxicology, 37, 13-22.
http://www.sciencedirect.com/science/article/pii/S027869159800101X
Bradford, P.G. & Awad, A.B. (2007). Phytosterols as anticancer compounds. Mol. Nutr. Food, Res., 51, 161–170.
http://onlinelibrary.wiley.com/doi/10.1002/mnfr.200600164/abstract
EU Commission Regulation 608/2004/EC concerning the labelling of foods and food ingredients with added phytosterols, phytosterol esters, phytostanols and/or phytostanol esters.
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:097:0044:0045:EN:PDF
EU Commission Regulation (EU) No 718/2013 of 25 July amending Regulation (EC) No 608/2004 concerning the labelling of foods and food ingredients with added phytosterols, phytosterol esters
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:201:0049:0050:EN:PDF
EU Scientific Committee on Food (2000). Opinion on a request for the safety assessment of the use of phytosterol esters in yellow fat spreads.
http://europa.eu.int/comm/food/fs/sc/scf/out56_en.pdf
EU Scientific Committee on Food (2002a). General view on the long-term effects of the intake of elevated levels of phytosterols from multiple dietary sources, with particular attention http://europa.eu.int/comm/food/fs/sc/scf/out143_en.pdf
EU Scientific Committee on Food (2002b). Opinion on a report on Post Launch Monitoring of “yellow fat spreads with added phytosterol esters”.
http://europa.eu.int/comm/food/fs/sc/scf/out144_en.pdf
EU Scientific Committee on Food (2003a). Opinion on applications for approval of a variety of plant sterol-enriched foods.
http://europa.eu.int/comm/food/fs/sc/scf/out174_en.pdf
EU Scientific Committee on Food) (2003b). Opinion on an application from ADM for
approval of plant sterol-enriched foods.
http://europa.eu.int/comm/food/fs/sc/scf/out192_en.pdf
EU Scientific Committee on Food (2003c). Opinion on an application from MultiBene for approval of plant sterol-enriched foods.
http://europa.eu.int/comm/food/fs/sc/scf/out191_en.pdf
European Food Safety Authority (2003). Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies. EFSA Journal, 15, 1-12 .
http://www.efsa.eu.int/science/nda/nda_opinions/216/opinion_nda_01_en1.pdf
European Food Safety Authority (2008). Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on “Plant Sterols and Blood Cholesterol: Scientific substantiation of a health claim related to plant sterols and lower/reduced blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA Journal (2008) 781, 1-12.
http://www.efsa.europa.eu/fr/scdocs/doc/781.pdf
Hallikainen M A et al (2000). Plant stanol esters affect serum cholesterol concentrations of hypercholesterolemic men and women in a dose dependent manner. Journal of Nutrition, 130, (4), 767-76.
http://www.adaevidencelibrary.com/worksheet.cfm?worksheet_id=250505&auth=1
Hendriks H F J et al (1999). Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. European Journal of Clinical Nutrition, 53, 319-327.
Hepburn P A et al(1999). Safety evaluation of phytosterol-esters. Part 2: Subchronic 90-day oral toxicity on phytosterol-esters — a novel functional food. Food and Chemical Toxicology, 37, (4), 521-532.
http://www.sciencedirect.com/science/article/pii/S027869159800101X
Jones P J H et al (2000). Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. Journal of Lipid Research, 41, 697-705.
http://www.enduranceresearch.com/media/Phytosterol_vs_Stanols.pdf
Katan et al (2003). Review “Efficacy and Safety of Plant Stanols and Sterols in the
Management of Blood Cholesterol Levels” for the Stresa Workshop Participants.
Mayo Clin Proc, August 2003, 78, 965-978.
http://www.mayoclinicproceedings.com/content/78/8/965.full.pdf
Law M (2000). Plant sterol and stanol margarines and health. British Medical Journal, 320, 861-864.
http://www.bmj.com/content/320/7238/861.full
Li J.H et al (2001) Measurement variability of plasma ß-sitosterol and campesterol, two new biomarkers for cancer prevention. Europ. J. Cancer Prev. 10, 245-249.
http://journals.lww.com/eurjcancerprev/Abstract/2001/06000/Measurement_variability_of_plasma.7.aspx
Lea, L.J. & Hepburn, P.A. (2006) Safety evaluation of phytosterol-esters. Part 9: Results of a European post-launch monitoring programme. Food Chem. Toxicol., 44, 1213–1222.
http://www.sciencedirect.com/science/article/pii/S0278691506000214
Mellanen P et al (1996). Wood-derived estrogens: Studies in vitro with breast cancer cell lines and in vivo in trout. Toxicology and Applied Pharmacology, 136, 381-388.
http://www.mendeley.com/research/woodderived-estrogens-studies-vitro-bre...
Normén L et al (2000). Soy sterol esters and ß-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. American Journal of Clinical Nutrition, 71, 908-13.
http://www.ajcn.org/content/71/4/908.short
Nguyen T T et al (1999). Cholesterol-lowering effect of stanol ester in a US population of mildly hypercholestrolemic men and women: a randomised controlled trial. Mayo Clinical Proceedings, 74 (12) 1198-1206.
http://www.mayoclinicproceedings.com/content/74/12/1198.full.pdf
Pollak 0 J (1953). Reduction of blood cholesterol in man. Circulation, 7, 702-706.
http://circ.ahajournals.org/content/7/5/702.full.pdf
PolIak 0 J and Kritchevsky, D (1981). Sitosterol. Monographs on atherosclerosis. Basel: S. Karger.
Sanders D J et al (2000). The safety evaluation of phytosterol-esters Part 6. The comparative absorption and tissue distribution of phytosterols in the rat. Food and Chemical Toxicology, 38 (6) 485-491.
Turnbull D et al (1999). 13 Week oral toxicity study with stanol esters in rats. Regulatory Toxicology and Pharmacology, 29, 216-226.
http://www.mendeley.com/research/13week-oral-toxicity-study-stanol-esters-rats/
Turnbull D et al (1999). Short term tests of estrogenic potential of plant stanols and plant stanol esters. Regulatory Toxicology and Pharmacology, 29, 211-215.
UK Food with Added Phytosterols or Phytostanols (Labelling) (England) Regulations 2004.
Waalkens-Berendsen D H et al(1999). Safety evaluation of phytosterol-esters. Part 3: Two-generation reproduction study in rats with phytosterol esters — a novel functional food. Food and Chemical Toxicology, 37, 683-696.
http://www.sciencedirect.com/science/article/pii/S0278691599000563
Weststrate J A and Meijer G W (1998). Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. European Journal of Clinical Nutrition, 52, 334-343.
http://www.ncbi.nlm.nih.gov/pubmed/9630383/
Weststrate J A et al (1999). Safety evaluation of phytosterol esters. Part 4: Faecal concentrations of bile acids and neutral sterols in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food and Chemical Toxicology, 37, 1063-1071.
http://www.sciencedirect.com/science/article/pii/S0278691599001027
Whittaker M H et al (1999). Two generation reproductive toxicity study of plant stanol esters in rats. . Regulatory Toxicology and Pharmacology, 29, 196-204.
Woyengo T A et al (2009). Anticancer effects of phytosterols. European Journal of Clinical Nutrition 63, 813–820.
http://www.nature.com/ejcn/journal/v63/n7/abs/ejcn200929a.html
World Health Organisation (2009). Safety evaluation of certain food additives / prepared by the sixty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Phytosterols, phytostanols and their esters, pp 117-164.
http://whqlibdoc.who.int/publications/2009/9789241660600_eng.pdf
************************************************************************************************
The Institute of Food Science & Technology (IFST) is the independent professional qualifying body for food scientists and technologists. It is totally independent of government, of industry, and of any lobbying groups or special interest groups. Its professional members are elected by virtue of their academic qualifications and their relevant experience, and their signed undertaking to comply with the Institute's ethical Code of Professional Conduct. They are elected solely in their personal capacities and in no way representing organisations where they may be employed. They work in a variety of areas, including universities and other centres of higher education, research institutions, food and related industries, consultancy, food law enforcement authorities, and in government departments and agencies. The nature of the Institute and the mixture of these backgrounds on the working groups drafting IFST Information Statements, and on the Scientific Committees responsible for finalising and approving them, ensure that the contents are entirely objective. IFST recognises that research is constantly bringing new knowledge. However, collectively the profession is the repository of existing knowledge in its field. It includes researchers expanding the boundaries of knowledge and experts seeking to apply it for the public benefit.
Competence, integrity, and serving the public benefit lie at the heart of IFST philosophy. At all times IFST aims to:
Benefit the public supply of safe, wholesome, nutritious, tasty and attractive food through the application of sound science and technology;
Improve public knowledge and awareness of important issues relating to the supply, production, safety and quality of food;
Develop and communicate the knowledge underlying food science and technology, and further the education of food scientists and technologists;
Safeguard the public by defining, promoting, and upholding professional standards of competence, integrity and ethical behaviour; and
Maintain these standards by encouraging members to continue their professional education and development throughout their careers.
In serving the public benefit IFST takes into account the many elements that are important for the efficient and responsible supply, manufacture and distribution of safe, wholesome, nutritious, and affordable foods with due regard for the environment, animal welfare and the rights of consumers.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert interpretation and opinion, on important food-related issues.
