
Updated September 2023
Leaves of the stevia plant (Stevia rebaudiana Bertoni) are the source of steviol glycosides, a group of high-potency, zero-calorie sweeteners. Permitted as sweeteners in the EU since December 2011, steviol glycosides offer an opportunity to reduce the added sugar in a range of foods and beverages without loss of sweetness or reliance on synthetic sweeteners. Although steviol glycosides may not be called “natural” in the EU, food labels often draw attention to glycosides’ origin in the leaf.
Most ingredient-grade glycosides are extracted from stevia plants, but some, such as the excellent-tasting rebaudiosides D and M, are only minor components of the leaf. Commercial supply of the latter glycosides currently relies on biotechnological techniques. These include fermentation with a genetically-modified yeast, or biotransformation, where common glycosides from leaf are enzymically changed into the desired product. This process should not be confused with “enzymic modification”, a more random treatment that results in materials that are not nature-identical, are not EU-recognised steviol glycosides, and are sold as flavour and sweetness enhancers.
Early stevia introductions depended on high contents of rebaudioside A. This glycoside, in common with several others, can exhibit undesirable side tastes of bitterness and liquorice. Modern blends of leaf extracts have greatly improved on rebaudioside A, while rebaudiosides D and M are exciting much interest because of their substantial freedom from side tastes.
Steviol glycosides have been examined by various toxicological and regulatory authorities and found safe at designated levels. EU regulations govern the use of these sweeteners and specify maximum concentrations allowed for each permitted application. The text below gives details. The regulations largely limit steviol glycosides in soft drinks (their main market) such that they have to be combined with other sweeteners to achieve normally accepted intensities of sweetness. To preserve a natural image, sugars are used to supplement the glycosides’ sweetness, giving rise to “reduced sugar” rather than “sugar free” products. In countries without use limits, such as the USA, much greater degrees of sugar reduction are possible than in the EU.
Steviol glycosides are stable to all food processing except where prolonged high temperature treatments in markedly acid environments are used. The sweeteners are stable in finished products.
Abbreviations
ADI Acceptable Daily Intake
EFSA European Food Safety Authority
EU European Union
FDA Food and Drugs Administration (of the United States)
GRAS Generally Recognized As Safe
HPS High Potency Sweetener
JECFA Joint Expert Committee (of the World Health Organisation and the Food and Agriculture Organisation of the United Nations) on Food Additives
SE Sucrose Equivalent
QS Quantum Satis
Until recently, synthetic high-potency sweeteners (HPS), such as aspartame and sucralose, were the only practical solution to the problem of removing sugars from food and drinks without loss of sweetness.
Steviol glycosides (E960) were added to the European Union (EU) list of permitted sweeteners in 2011. This meant that, for the first time, there was available a non-caloric HPS, derived from nature, that had a taste quality good enough for it to be a significant source of sweetness in soft drinks and other products. This statement considers the origin, taste quality, regulation and practical application of steviol glycosides.
The shrub Stevia rebaudiana is a member of the Asteraceae and native to Paraguay, where the leaves have a history of use as a sweetener for over a century (Kinghorn 2002). Uniquely, the plant secretes in its leaves a group of structurally-related high-potency sweeteners, the steviol glycosides. All are ent-kaurene diterpenoid glycosides with a common core of steviol (ent-13-hydroxykaur-16-en-19-oic acid - see Figure 1) to which sugar groups are attached.
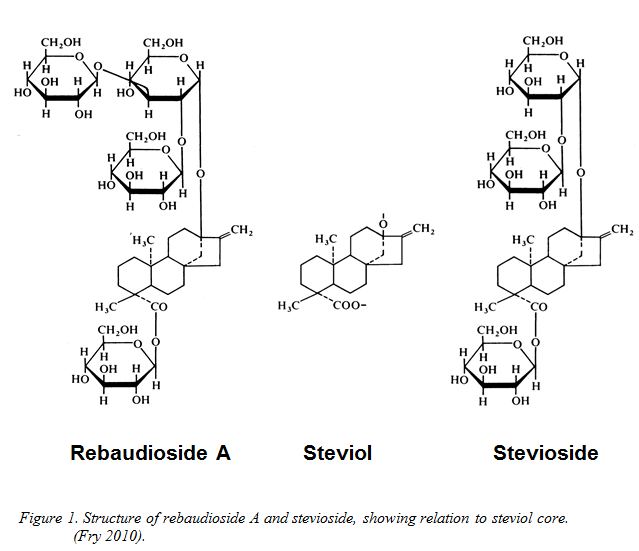
The most abundant of these molecules in the wild-type plant is stevioside, but the less-plentiful rebaudioside A (reb A) is widely held to be the better tasting of the two (Fry 2012; Phillips 1987). There are many other glycosides in the leaf, but generally at minor to trace concentrations. Because of the taste preference for reb A, conventional plant breeding programmes have been directed over many years to increasing the yield of this compound. Dried leaves of Stevia rebaudiana typically contain about 7-15% by weight steviol glycosides (Carakostas et al. 2011). Of this, 40% might be reb A in a modern cultivar (Morita and Bu 2000a, 2000b; Morita et al. 2009). More recently some of the minor glycosides, such as reb D and reb M, have been found to have superior taste quality. Various routes to providing these glycosides in commercial quantities are described later.
Stevia is widely cultivated in South America, but much of the world's commercial supply currently originates in Asia. There has also been interest in growing the crop in numerous other locations, including Canada, Australia, the USA, Southern Mediterranean countries, Thailand and Africa.
The glycosides are extracted by steeping dry leaves in warm water, filtering the extract free of solid material, and purifying the resultant solution by a combination of chromatography and crystallisation from water-alcohol solutions. This results in a crude extract comprising a mixture of glycosides in variable proportions, generally containing predominantly stevioside and rebaudioside A. Such extracts may be used directly but are normally further refined by additional recrystallisation to create ingredient-grade HPS that meet the purity requirements of Western markets.
Leaf extracts
The first ingredient-grade product launched in Western markets was essentially pure reb A. Lower-cost extracts containing less reb A quickly followed. These are often described by their reb A content such as “RA80” or “RA50”, meaning a minimum of 80 or 50% reb A respectively. Contrary to expectations, extracts that were not pure reb A but which had high reb A content (say around RA70 and above) were found to be of similar sweetness potency to pure reb A, but with a superior taste quality. This observation inspired far-reaching investigations of the taste qualities of individual glycosides (Hellfritsch et al. 2012) and their interactions when blended (Carlson et al. 2015). The insights thus afforded are the basis of a current generation of stevia ingredients where leaf extracts are carefully blended to maximise potency while reducing unwanted side-tastes such as bitterness or liquorice. Some extracts are blended for specific applications such as dairy or soft drinks.
Enzyme-modified stevia
Alongside these developments, suppliers have looked to earlier Japanese studies involving enzymic modification of steviol glycosides to improve taste quality. It is a general rule that the greater the number of sugar groups attached to the steviol nucleus, the better the sweetness quality. Enzyme modification increases the number of substituent sugar groups by incubating a low-cost extract (usually high in stevioside) with a glucosyl transferase enzymes and a source of glucose. The original plant glycosides emerge modified by the addition of one to three (or more) extra glucose residues. The exact glycoside composition that results is often indeterminate and the modified glycosides are not nature-identical. The material usually also contains some unreacted glucose and a small quantity of unreacted feedstock glycosides. Enzyme modified stevia (also called glycosylated steviol glycosides) does not meet EU regulatory requirements for sweetener-grade steviol glycosides and is primarily sold to flavour houses as a flavouring and sweetness enhancer.
Fermentation
Further study of minor glycosides from the leaf has focused attention on compounds such as reb D and reb M. These have a much higher quality of sweetness than reb A, or even controlled blends of leaf extracts, and have excited much interest, particularly reb M (Prakash, Markosyan & Bunders, 2014). The drawback of such minor glycosides is that they exist in the leaf at such low concentrations that their isolation from this source is currently prohibitively costly. Three approaches are being taken to produce commercial quantities economically. The first is to use a fermentation process based on genetically modified yeast. The glycosides produced are identical to those in the stevia leaf and would be regarded as natural for regulatory purposes in the USA - not the case in the EU. Accordingly, while such products appeared commercially in the USA in 2018, their use in the EU is likely to be more delayed.
Biotransformation
This second approach uses enzymes to change glycosides extracted from leaves into reb D and reb M. This differs from “enzyme modification” above in that the enzymes are highly specific and create nature-identical reb D and reb M. Commercial supplies of these biotransformed glycosides were beginning to become available in 2018.
Plant breeding
The final route to obtaining minor glycosides relies on conventional plant breeding to enhance yields in stevia leaf. This is the same process used successfully to increase yields of reb A in the past. Even if feasible for reb D and reb M, this is likely to be a long-term process.
|
Modern investigations, including human studies on safety, metabolism and intake, fully support the safety of stevia sweeteners. Stevia has been the subject of biological and toxicological investigations for more than 50 years. The earliest of these studies were conducted with crude or poorly defined test materials. These historical results were both unacceptable to modern toxicologists, and the source of some confusion about actual safety. Both matters were eventually resolved by work conducted with fully characterised, high-purity reb A extracts (Brusik 2008). This led to the USA FDA issuing a letter of “no objection” to the designation of reb A as “Generally Recognized As Safe” (GRAS) in December 2008. The GRAS designation was subsequently accepted for extracts containing other steviol glycosides (Carakostas et al. 2008). Elsewhere, JECFA thoroughly reviewed the data on steviol glycosides and concluded that they are safe for use in food and beverages for people of all ages and populations. JECFA first set tentative purity specifications in 2004 and published a final version in 2010 (JECFA 2010). An Acceptable Daily Intake (ADI)[1] of 4 mg/kg body weight /day (expressed as steviol) was established in 2008 and remains current. This compares with 40 mg/ kg for aspartame, 5 mg/ kg for saccharin and 15 mg/ kg sucralose. To put these figures in context, 4 mg/kg as steviol equates to 12 mg/kg of rebaudioside A. This means for the average UK 9-10 year old child (weighing 30kg) the ADI would be 360 mg of rebaudioside A, an amount provided by 1.5 litres of soft drink sweetened at the EU maximum permitted concentration. A 70kg adult would need to consume 3.5 litres of such a drink to reach the ADI for rebaudioside A. In 2010 the European Food Safety Authority (EFSA) concluded that steviol glycosides are safe and established the same ADI as JECFA. This resulted in a European regulation permitting steviol glycosides in a range of food and drink from December 2011 (European Commission, 2011[2]). Today, there are a large number of studies on stevia in the scientific literature that corroborate the safety conclusions of regulatory agencies around the globe. Table 1 includes a list of safety reviews and regulatory approvals that have enabled growth of the stevia sweetener market. Steviol glycosides are approved for use in many other countries, including Canada, the EU, Korea, Mexico, Taiwan, China, Russia, Australia, Argentina, New Zealand, Colombia, Peru, Uruguay, Brazil, Japan and Malaysia. EU regulatory position - composition The EU recognises 11 steviol glycosides (European Commission, 2016) and currently requires that any commercial ingredient be a minimum 95% of any combination of the recognised glycosides. Up to 5% non-steviol glycoside material is permitted. In practice, this fraction usually comprises non-EU-recognised steviol glycosides. However, at the time of writing, EFSA has declined to endorse a proposal that any steviol glycoside found in the leaf may be included in the 95% requirement (EFSA, 2018). The breadth of this specification means there is a wide range of ingredient compositions available on the market, extending from pure single glycosides (mainly reb A and the cheaper stevioside) to customised extracts that contain considerable quantities of other EU-recognised steviol glycosides. All can be designated E960 in the EU. Table 1. Global safety reviews for steviol glycosides
EU regulatory position - use levels The permission to use this sweetener across the EU is defined by category and addition rates limited by steviol equivalence, all outlined in Commission Regulation No. 1131/2011 (Table 2) and an amendment for mustard (European Commission, 2016a). Other categories may be added in future. Table 2. Maximum level of steviol equivalents in specific categories
EU regulatory position - steviol equivalents
The reason for expressing use limits as steviol equivalent is that the steviol nucleus is the only part of the sweeteners that is of toxicological interest; the attached sugar residues are toxicologically irrelevant. Unfortunately, because the glycosides differ in structure and molecular weight, the legally-recognised glycosides each contribute different amounts of steviol (see Figure 1). As a result, manufacturers need to know the steviol equivalent of the particular mixture of glycosides they are using. From the manufacturers’ point of view, it is highly desirable that this figure does not change from lot to lot as, in the worst case, this could necessitate reformulation to avoid exceeding statutory limits. Consistency of ingredient supply is thus critical. To calculate the steviol equivalence of a particular stevia extract the proportions of the various components must be known. Steviol equivalent concentration is the product of the conversion factor and the concentration of the specific steviol glycoside concerned. The EU-approved conversion factors are shown in Table 3. They occasionally differ to a minor degree from those applied by other countries such as Australia and New Zealand. Table 3 Conversion factors
*European Commission, 2012, 2016b
|
As with all high-potency sweeteners, each steviol glycoside’s concentration–sweetness response behaviour is a curve tending to a maximum value of intensity, expressed in % sucrose equivalent (% SE). In other words, the potency of the sweeteners (in terms of the number of times they are as sweet as sucrose) is non-linear and depends on the concentration used. There is no agreed standard concentration, but values around 5% SE are realistic indicators and often used. The potency of reb A in room temperature water at 5% SE is 250 (Fry, Yurttas & Biermann, 2011), but that of stevioside is only half this (DuBois et al. 1991). The latter glycoside is thus at an immediate disadvantage in that twice as much is required to reach the same sweetness intensity as a given concentration of reb A (Figure 2). To compound this disadvantage, regulatory reasons mean the maximum amount of stevioside that can be used in any EU application is substantially lower than that of reb A. (See conversion factors Table 3 and Figure 4).
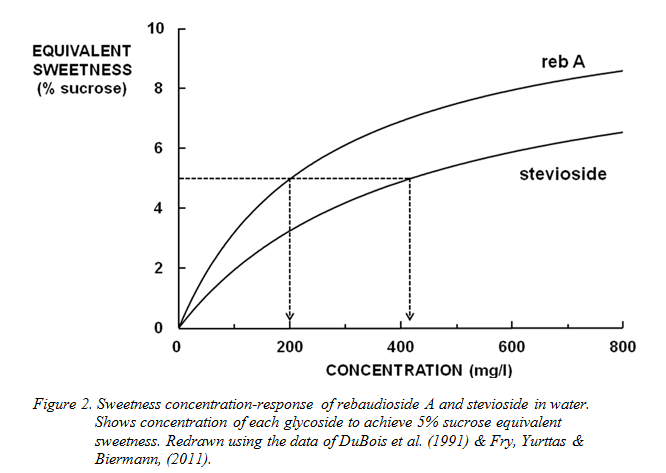
In addition, the quality of sweetness available from stevioside is significantly inferior to that from reb A. Both compounds have inherent non-sweet side tastes in addition to
sweetness, but reb A is widely regarded as the better-tasting of the two (Phillips 1987; Fry 2012). Control of these side tastes is key to successful product formulation. This encourages formulators to use the lowest practicable concentration of the sweeteners because side tastes are disproportionately less apparent at modest concentrations than at higher levels.
The maximum sweetness available is, however, limited. For example, in soft drinks, EU legal restrictions will mean that, even using pure reb A, the highest sweetness achievable will be about 5–6%SE (Figure 3). In contrast, most carbonated soft drinks contain around 10% sucrose. Accordingly, steviol glycosides are blended with other sweeteners except where a product, such as a flavoured water, is acceptable with a low sweetness intensity.
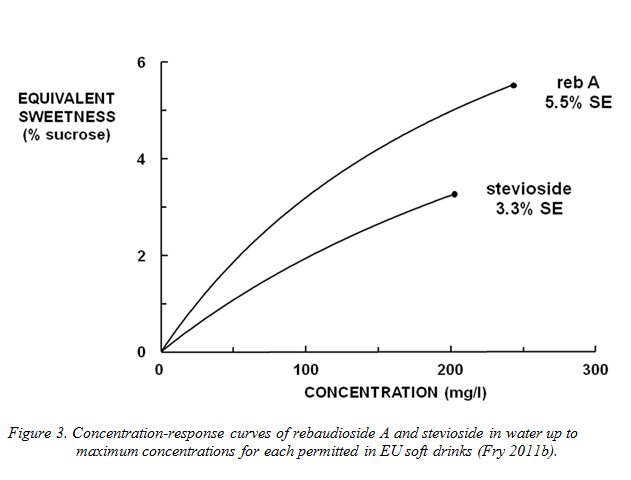
While it would be legal to blend with synthetic high-potency sweeteners (e.g. aspartame, sucralose and the like) the preferred candidates for blending are sugars, as these allow a natural ingredient image to be retained. As a result, the EU regulations effectively oblige makers to produce soft drinks with some sugar, and most stevia-sweetened soft drinks are ‘reduced sugar’ rather than ‘sugar-free’.
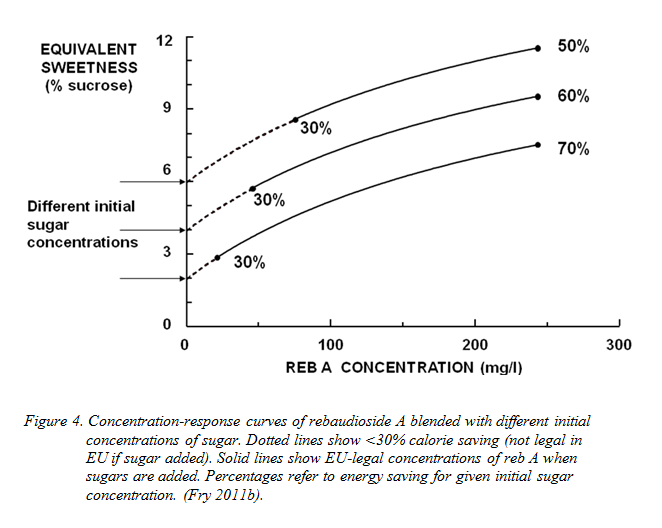
This development has been encouraged by the fact that the steviol glycosides taste remarkably good in combination with low concentrations of sugars. Figure 4 illustrates the use of reb A in conjunction with different background concentrations of sugar - and some of the EU regulatory constraints on this.
Sucrose is not the only source of sweetness that works well with steviol glycosides, and sugars from fruit, syrups such as honey, agave and fructo-oligosaccharides, and sweetness from sugar alcohols all serve too.
While non-alcoholic drinks represent the largest market opportunity for any high-potency sweetener, the steviol glycosides are suited to much wider application. Dairy products, particularly yoghurt, work well and European formulators have already turned their attention to this as well as ice cream and flavoured milks. Sugar-free confectionery is another successful application. Other categories include jams, jellies and fruit spreads. In the USA snack foods, alcoholic drinks and desserts in general also feature stevia sweeteners. Stevia-based table top sweeteners are particularly successful sugar substitutes on both sides of the Atlantic and are presented in powder, tablet or liquid form. They use a carrier to dilute the steviol glycosides so that consumers can readily dispense the sweetness equivalent to that of a teaspoon of sugar. This wide range of products is in part possible because of steviol glycosides’ high stability.
Reb A has been extensively studied and is stable to food processes such as, extrusion, pasteurisation, ultra-high temperatures, hot fill and canning. The sweetener is also very stable in baking (Fry 2011a, 2012) and although industrially-baked goods are generally not a permitted application for any high-potency sweetener in the EU, home baking has proved a popular way of using table-top sweeteners in the EU and especially the USA.
The variability of stevia composition means the taste of different extracts can differ widely. As previously noted, the taste of reb A is preferred to that of stevioside. However, these two major glycosides also differ in their effectiveness as sweeteners. Figure 2 contrasts the concentration-response behaviour of stevioside and rebaudioside A. As for all HPS, the concentration-response graph is, in each case, a hyperbolic curve. In contrast to sugars, all HPS become less effective at sweetening the higher their concentration. For this reason, potency figures should be accompanied by the sucrose equivalent intensity (SE) at which they were measured. In the case of reb A, its potency in water is 250 at 5% SE (Fry, Yurttas & Biermann, 2011). Figure 2 shows that rebaudioside A is twice as potent as stevioside. Thus, reb A is not only preferred for its taste, but is also a more effective sweetener on a weight basis than stevioside.
All sweeteners differ in their temporal (intensity/time) responses too. Steviol glycosides, in common with other HPS, can exhibit slightly delayed onset of sweetness and some glycosides have a tendency to linger. These qualities vary with glycoside composition and mean each version of stevia has its own challenges when optimising the taste of consumer products.
Steviol glycosides exhibit tastes in addition to sweetness. These non-sweet side tastes include sensations commonly described as bitter and liquorice. These tastes are intrinsic to steviol glycosides and cannot be eliminated by further refining. However, the side tastes of reb A, and mixtures rich in reb A, are generally only apparent at relatively high concentrations and are not significant at lower levels (Prakash et al. 2008). Nevertheless, some flavours (such as citrus and mint) and systems (such as tea-based beverages and alcoholic drinks) are remarkably acceptable with higher concentrations of steviol glycosides. The optimum amount to use is application-dependent and normally located by trial-and-error.
As noted earlier, there is a trend to use leaf extracts containing lower amounts of reb A, blended to maximize beneficial taste synergies between different glycosides. Such blends provide superior sweetness quality to reb A alone or high reb A extracts, especially at higher sweetness intensities. Some suppliers are now developing steviol glycoside mixes particularly suited to specific applications, such as dairy. These improvements are more advanced in the USA, which is free of use limits.
Minor glycosides reb D and M are exciting interest because they have very low side tastes and a much cleaner sweetness than other glycosides or blends. Where regulations do not limit the amount used, these glycosides allow for much greater substitution of sugars, possibly even to the extent of producing a true zero-calorie beverage sweetened exclusively with stevia to an intensity around 10%SE.
A key advantage of the steviol glycosides is that they are seen by consumers as having been derived from nature. Accordingly, despite the limited sweetness intensity allowed by EU regulation, there is some reluctance to blend them with synthetic sweeteners in order to achieve zero or near-zero calorie beverages. The main use of the glycosides is thus in combination with caloric sweeteners in drinks of reduced sugar and/or energy content.
Figure 4 illustrates the effects of such blending with various different concentrations of sugars. The sweetness of the steviol glycosides is additive to that of sugars and the overall sweetness available is thus boosted by the contribution of the background sugar level. This blending permits customary degrees of sweetness to be reached without difficulty. However, in products that have added sugars there is still the EU regulatory requirement to achieve a minimum 30% calorie saving. This is shown in Figure 4 by dotted portions of the concentration-response curves. It would not be legal to make drinks with the composition shown by the dotted lines, as the calorie saving would be insufficient. In contrast, the solid lines describe fully-legal potential compositions, and it can be seen that not only can the 30% requirement be met readily, but much higher calorie reductions are also attainable within the regulatory limits.
The success of this approach can be seen in the USA, where a number of major brands enjoy substantial sales of juice-based beverages, partly sweetened with reb A, generally claiming about 50% lower energy contents on their labels and ranging in juice content from 24% to 75%.
Another trend seen in the USA is the use of erythritol in beverages in combination with steviol glycosides. Erythritol performs exceptionally well in improving the sweetness quality of stevia and has the advantage of being zero-calorie. Up to 3.5% erythritol is permitted in soft drinks in the USA, although generally lower concentrations are used. The EU permits erythritol in non-alcoholic drinks at up to 1.6% as a flavour enhancer (European Commission, 2015).
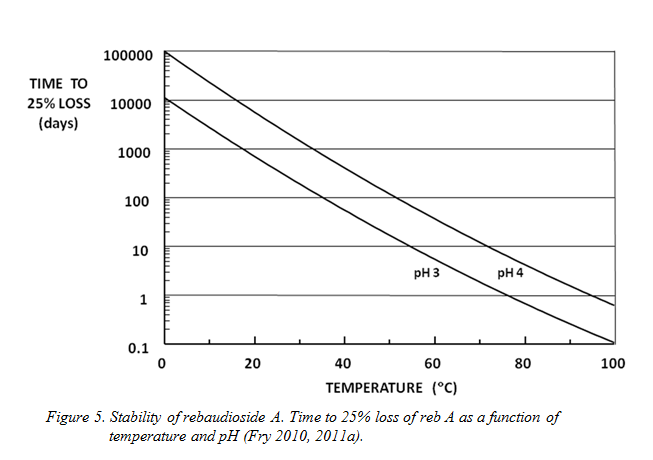
Practical application of the steviol glycosides requires that they are sufficiently stable to provide adequate product shelf life. The glycosides’ stability depends on pH, temperature and time. The effect of these variables is shown in Figure 5, which demonstrates how the time needed for a specified degree of reb A breakdown (25%) to occur depends on temperature for the pH 3-4 range typical of most soft drinks.
The 25% loss figure is a common industry guideline for the maximum acceptable decline in sweetness in those drinks that employ the relatively unstable sweetener, aspartame. However, it should be noted that the breakdown products of reb A are other glycosides, most of which are themselves high-potency sweeteners. This means that a 25% analytical loss of reb A results in only about 7% drop in sweetness (Fry et al, unpublished), and that Figure 5 gives a highly pessimistic view of the sweetener’s shelf life in terms of taste. In addition, at refrigerated and room temperatures reb A is very stable indeed. Moreover, the sweetener is more than capable of withstanding the heat processing involved in treatments such as pasteurisation and hot fill as well as UHT and canning.
Steviol glycosides are non-caloric, high-potency sweeteners mostly extracted from the leaves of Stevia rebaudiana. The glycosides, permitted as sweeteners in the EU and widely elsewhere, are ideal for use alone or blending with caloric sweeteners in the formulation of a range of reduced sugar foods. The glycosides are stable to processing and storage. While some glycosides have non-sweet side tastes that limit their use, newer, minor glycosides have improved sweetness quality. Commercial supply of these, however, currently depends on making them biotechnologically, either by fermentation or by enzymic synthesis.
[1] The Acceptable Daily Intake (ADI) is defined as an estimate of the amount of a food additive, expressed on a bodyweight basis, that can be ingested daily over a lifetime without appreciable risk to health. "Without appreciable risk" means based on current knowledge, certainty that no harm will result, even after a lifetime of exposure to the additive concerned. The ADI is usually given as a range of 0-x milligrams per kilogram of bodyweight per day. Note the ADI is calculated using very conservative safety factors and is not regarded as a toxic limit. Occasional consumption in excess of the ADI is permissible.
[2] Note that this has subsequently been amended to ease compositional requirements and add reb M as a recognised glycoside (European Commission, 2016)
Carakostas, M., Prakash, I., Kinghorn, D., Wu, C. and Djaja, D.S. 2012. Steviol Glycosides. In: O’Brien Nabors (ed) Alternative Sweeteners 4th Edition. CRC Press, Boca Raton. https://www.routledge.com/Alternative-Sweeteners/OBrien-Nabors/p/book/9781138198562
JECFA, 2021. Framework for Steviol Glycosides. Specifications Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 91st Meeting 2021. https://www.fao.org/3/cb8031en/cb8031en.pdf
Kinghorn, A.D. 2002. Overview. In: Kinghorn, A.D. (ed.) Stevia: The Genus Stevia. Taylor & Francis, London. https://www.taylorfrancis.com/books/edit/10.4324/9780203165942/stevia-douglas-kinghorn
Prakash, I., DuBois, G. E., Clos, J. F., Wilkens, K. L. and Fosdick, L. E. 2008. Development of rebiana, a natural, non-calorific sweetener. Food Chem. Toxicol., 46, S75–82.
https://www.sciencedirect.com/science/article/abs/pii/S0278691508002329
Brusik, A. (ed) 2008. Rebaudioside A: An assessment of safety, Food Chem. Toxicol. 46, Supplement 7S.
Carakostas, M.C., Prakash, I., Kinghorn, A.D., Wu, C.D. and Soejarto, D.D. (ed.) 2011. Steviol Glycosides. CRC Press (Taylor & Francis), Boca Raton.
Carlson, T.L., Schmelzer, W.N., Tyler, C.A., Guthrie, B.D., Lindgren, T.A. and Mortenson, M.A., 2015, Stevioside Blends, USA Patent Application 20150237898.
DuBois, G.E., Walters, D.E., Schiffman, S.S., Warwick, Z.S., Booth, B.J., Pecore, S.D., Gibes, K., Carr, B.T. and Brands, L.M. 1991. Concentration-response relationships of sweeteners. In: Walters, D.E., Orthoefer, F.T. and DuBois, G.E., (eds.) ACS Symposium Series: Sweeteners. Discovery, Molecular Design and Chemoreception. American Chemical Society, Washington, DC. p 261–76.
EFSA, 2018. Safety of the proposed amendment of the specifications of the food additive steviol glycosides (E 960), EFSA Journal, 16(3):5236.
European Commission 2011. Commission Regulation (EU) 1131/2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with regard to steviol glycosides. Official Journal, L 295:205-211.
European Commission 2012. Commission Regulation (EU) 231/2012, Laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008. Official Journal, L83:270-217.
European Commission 2015. Commission Regulation (EU) 2015/1832 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Erythritol (E 968) as a flavour enhancer in energy-reduced or with no added sugars flavoured drinks. Official Journal, L 266:27-28.
European Commission 2016a. Commission Regulation (EU) 2016/441 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the use of Steviol glycosides (E 960) as a sweetener in mustard. Official Journal, L 78:47.
European Commission 2016b. Commission Regulation (EU) 2016/1814 Amending Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008. Official Journal 278:37.
Fry J.C. 2010. Stevia update. Presentation to the International Society of Beverage Technologists, Copenhagen, Sept 29-30.
Fry J.C. 2011a. Rebiana in baked goods. Cereal Food World 56:191–4.
Fry J.C. 2011b. Stevia use limits. Brake or blessing? Presentation to the International Society of Beverage Technologists, Vilvoorde, Sept 29-30.
Fry J.C., Yurttas N. and Biermann K.L. 2011. The sweetness concentration-response behavior of rebiana at room and refrigerator temperatures. J. Food Sci. 76(9):S545-8.
Fry, J.C. 2012. Natural low-calorie sweeteners. In: Baines, D. and Seal, R. (eds.) Natural Food Additives, Ingredients and Flavourings. Woodhead, Cambridge.
Hellfritsch, C., Brockhoff, A., Stähler, F., Meyerhof, W. and Hofmann, T., 2012, Human psychometric and taste receptor responses to steviol glycosides J. Agric. Food Chem., 60 (27): 6782–6793 †
JECFA 2010. Steviol Glycosides. In Compendium of Food Additive Specifications, FAO JECFA Monographs 10, FAO, Rome, pp17-23.
Kinghorn, A.D. 2002. Overview. In: Kinghorn, A.D. (ed.) Stevia: The Genus Stevia. Taylor & Francis, London.
Morita, T. and Bu, Y., 2000a. Variety of Stevia rebaudiana Bertoni. USA Patent 6 031 157.
Morita, T. and Bu, Y., 2000b. Variety of Stevia rebaudiana Bertoni. USA Patent 6 080 561.
Morita, T., Morita, K. and Komai, K., 2009. High rebaudioside-A plant. USA Patent Application 20090214753.
Phillips K.C. 1987. Stevia: steps in developing a new sweetener. In: Grenby T.H. (ed.) Developments In Sweeteners—3. Applied Science Publishers, London.
Prakash, I., DuBois, G. E., Clos, J. F., Wilkens, K. L. and Fosdick, L. E., 2008. Development of rebiana, a natural, non-calorific sweetener. Food Chem. Toxicol., 46, S75–82.
Prakash, I., Markosyan, A. and Bunders, C., 2014, Development of Next Generation Stevia Sweetener: Rebaudioside M, Foods, 3:162-175.
Institute of Food Science & Technology has authorised the publication of the following updated Information Statement on Stevia dated September 2023, replacing that of February 2019.
This updated Information Statement has been updated by Professor Julian Cooper FIFST and was originally prepared by John Fry FIFST, peer reviewed by professional members of IFST and approved by the IFST Scientific Committee.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
