
June 2021
It is pointless to send a sample for an analytical test if you do not know what you will do with the result. Interpretation of results, particularly for food authenticity verification tests, is not always straightforward. The interpretation can be as complex as the testing. The sampling protocol that was used is one key factor in the interpretation and explained in IFST Information Statement ‘Sampling for Food Analysis - key considerations’. Interpretation will depend upon the purpose of the testing, and upon the consequences of a ‘fail’ result. Each demands a different level of confidence. You would expect more confidence in a result that underpinned a decision to recall stock than a decision to reclean a conveyor belt.
Decisions that could be informed by test results |
||
|
|
Examples
|
|
|
Is a food or ingredient legally compliant?
|
Pesticide residues, heavy metals
|
|
|
Is it safe?
|
Microbiology, undeclared allergens,
|
|
|
Does it comply with quality specification?
|
Brix, hardness of biscuits
|
|
|
What figures to use on pack labels?
|
Nutritional testing
|
|
|
Is it what it claims to be?
|
Country of origin, ‘natural’ ingredients
|
|
|
What shelf life should I assign?
|
Stability studies
|
|
|
Should I change my cleaning process?
|
Factory cleaning verification swabs
|
|
|
Factory process optimisation?
|
In-process testing for acrylamide
|
|
|
Should I develop a new product?
|
Competitor product analysis
|
|
|
Should I trigger an unannounced audit?
|
Food authenticity tests
|
|
No analytical result is absolute, irrespective of the test method. All results have inherent associated uncertainty. There is uncertainty in the correct identification of the substance or parameter being measured, and also in quantifying the amount measured. It is important that you, as a laboratory’s customer, understand the significance of this uncertainty. If the test method is accredited to ISO 17025:2017 then your laboratory has an obligation to make you aware of the analytical uncertainty and its significance. If they are reporting a compliance decision (i.e. ‘PASS/FAIL’ against any limit or specification) then they also have an obligation to report how conservatively they have accounted for this uncertainty in reaching that decision. This is termed the ‘Decision Rules’ in ISO/IEC 17025:2017[1]
It is important that you have open communication with your laboratory. They need to understand the purpose and context of the testing, and you need to understand the uncertainty and caveats around the result and its interpretation.
There is a myriad of available analytical test methods. Overviews are given in the following IFST Information Statements: ‘Chemical Analysis - key considerations’, ‘Microbiological Analysis - key considerations’ and ‘Physical Analysis - key considerations’. One way of categorising methods is by the degree of certainty in the result. Methods with high confidence that a ‘negative’ result is truly negative (irrespective of whether a ’positive‘ result is truly positive) are used for cheap high-throughput screening tests. Methods, with high confidence, where a ’positive screening result’ is truly ‘positive’ are used for confirmatory testing, but even with the most confident confirmatory test, there is always a small, maybe negligible, analytical uncertainty that must be reported and contextualised. Sometimes it is necessary to carry out a range of confirmatory tests, using different analytical techniques, before the ’positive screening result’ can be confirmed. In some cases, the same method can be used for both screening and confirmation. The degree of confidence depends on how the method is calibrated and the associated quality controls that run.
Some examples are given in the table below:
Application example |
Desired confidence |
Example method |
||
|
Negative screening results |
Positive screening results |
Confirm/ quantify ’positive screening’ results |
||
|
Screening - antibiotic residues in milk |
High |
Low |
Low |
Inhibitor test kits |
|
Screening - allergens |
High |
Medium |
Low |
Lateral flow kits |
|
Total fat content -online testing for meat specification |
Medium |
Medium |
Medium |
Low-resolution NMR |
|
Confirmation of allergens |
High |
Medium |
Medium |
None available. ELISA is used but does not give high confidence in confirmation of identity |
|
Nutritional label - total fat content |
High |
Medium |
Medium |
Gravimetric tests |
|
Unlabelled meat species in a meat product |
Medium |
High |
Low/Medium |
Species-specific DNA testing by PCR or NGS DNA testing |
|
Undeclared sugars or syrups |
High |
High |
Low |
FT-IR or high-resolution
|
|
Complaint investigation – taint or flavour compounds |
High |
High |
High |
GC-MS/(MS)
|
|
Legal/safety compliance - process contaminants |
High |
High |
High |
LC-MS/(MS) of process contaminants
|
If a single test method cannot achieve the required level of confidence, as is often the case with allergens, then two different test methods may be run.
The exception to this categorisation is in the field of food authenticity testing. Analytical techniques are sometimes configured to give an ‘is it typical?’ result, rather than a ‘pass/fail’, by using multivariate statistics to match against a database of authentic samples. In these cases, confidence in the result is driven primarily by the construction of the ‘authentic’ database and by the statistical algorithms, rather than by the analytical technique. This is covered in IFST Information Statement ‘Food Authenticity Testing Part 1: The Role of Analysis’.
Some analytical results, e.g. meat content by nitrogen factors, are derived by calculation, using the results of a series of different (indirect) tests. This makes estimation of the uncertainty associated with the final result much more difficult.
It is important to remember that the sampling protocol (see IFST Information Statement ‘Sampling for Food Analysis - key considerations’) usually has a bigger impact on measurement uncertainty than the analytical test method.
Repeating the test will generally increase the certainty of the result. It is important to know if your result is based on a single test, or if it is the average of repeat testing.
- legal limits
- international standards e.g. ISO, Codex
- industry guidelines or recommendations
- business to business (B2B) Codes of Practice, customer specifications, trading terms
- internal specifications
- absence e.g. for evidence of fraudulent origin.
| Example The EC Food Information for Consumers Regulations (EC/1169/2011) allow a tolerance on the labelled content of macro- and micronutrients (typically 30%) to allow for measurement uncertainty and product variability. |
Your laboratory needs to understand which specification you are testing against, and what expectations you have regarding the tolerance. This is particularly important if the laboratory is in a country where different specifications may apply. For example, unless told otherwise, laboratories in Germany or Belgium would test mineral oils (a contaminant that can migrate into food from recycled cardboard packaging, lubricating oils, or engine smoke) against compliance with their own national guidelines, whereas very few UK food companies believe that a fixed specification is appropriate.
Even once a specification is agreed, accredited laboratories are obliged to agree with you in advance about how they will account for measurement uncertainty, when certifying compliance. This is irrespective of whether the specification already has an accepted tolerance, e.g. nutritional labelling, or whether it is mandatory, e.g. maximum residue limit (MRL), or ISO safety criteria. How conservative you are about the treatment of measurement uncertainty depends upon your appetite for risk (Figure 1). An accredited laboratory is required to understand this, and to agree ‘Decision Rules’ with you whenever they are to report a verdict against compliance criteria. They will usually be obliged to use a guard band to adjust the acceptance limit[1]. In this case, you must agree as to how conservative the guard band is, and whether the guard band is set to err on the side of the consumer PAH (acceptance limit), or the producer (rejection limit) - see Figure 1(b).
Figure 1(a): Ways to account for uncertainty in decision rules
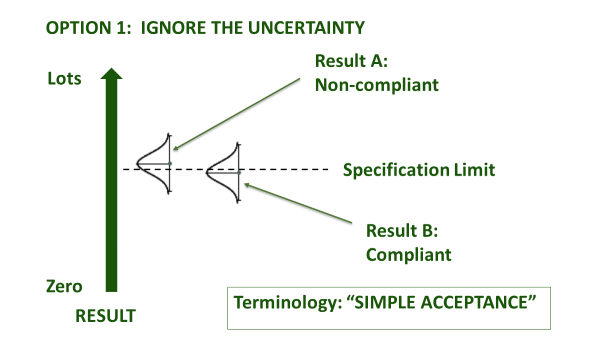
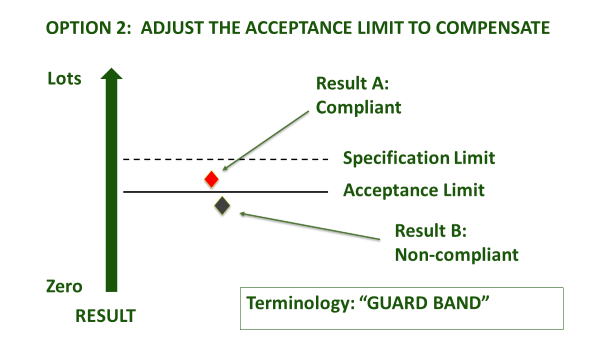
Figure 1(b): Ways to use a guard band
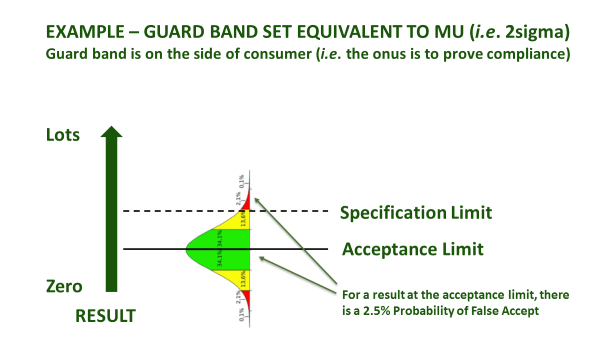
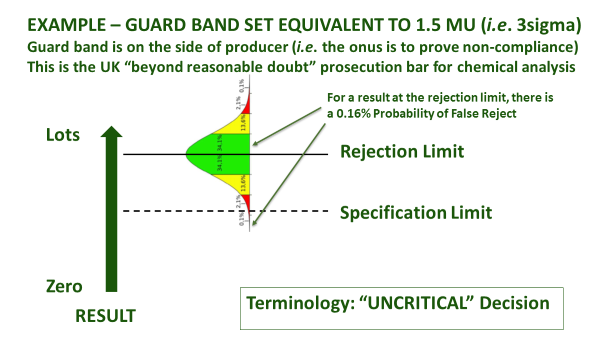
These concepts of decision rules and statements of conformity do not fit well with those authenticity tests that are based upon a ‘match against normality’, but if laboratories are ISO 17025 accredited then they must still discuss and agree them with you. Rather than ‘compliant’ or ‘non-compliant’, the result would best be expressed as a probability that your sample ‘matches an authentic population’ or ‘does not match an authentic population’. Unfortunately, most statistical algorithms do not allow the calculation of a numeric probability fit. Laboratories use phrases like ‘suspicious’ and ‘not consistent with ... ‘, on their certificates. There are some cultural variations so German laboratories, for example, tend to be much more black-and-white in their certificate phrasing than UK laboratories. It is important that you understand what these phrases mean, and any caveats that lie behind them.
Even once the decision rules have been agreed, and a Certificate of Analysis is provided, it does not automatically follow that a non-compliant analytical result means that your sample is unfit (for use or sale). There are a number of factors that affect the extrapolation from the result of the laboratory sample to interpreting the condition, legality or quality of your product. These include the following factors:
- best- and worst-case assumptions - effect of sampling and sub-sampling variation
- whether the laboratory sample was processed in the same way as the specification was expressed. For example, is the nutritional specification, and testing, expressed as how it is sold or consumed? Did the laboratory follow the on-pack cooking instructions?
- the fact that there are sometimes different definitions and ways of expressing the same parameter e.g. dietary fibre (see IFST Information Statement ‘Dietary fibre’). Did the laboratory use the same definition as your specification so that they are comparing like-for-like?
- what are the best- and worst-case assumptions of toxic risk, for contaminants or allergens (where there is no regulatory limit), accounting for portion sizes, vulnerable population groups etc. Is the contaminant a ‘known risk’ or something that an informed consumer would reasonably expect in your product, and consider acceptable, e.g. PAHs in chargrilled food
- are there alternative hypotheses - innocent explanations why a sample may not match a reference database? For example, your ingredients being grown in a novel fertiliser, or meat sourced from animals with an unusual feed ration
- are there alternative hypotheses - sources of contaminants other than the source with a regulated limit? For example, anthraquinone in tea leaves is far more likely to arise as a process contaminant from the drying process than from pesticide use on a tea plantation.
The five examples below raise just some of the issues that must be clarified between you, as the customer, and your laboratory before you commission a test, in order that you know in advance what you will do with the results.
1. Nutritional label verification and meat content for a packet of ‘low salt’ bacon
|
There are many ways to interpret results and to judge compliance. All must be agreed, in detail, with your laboratory before testing. There is no longer a legal tolerance on analysis of meat content versus labelled quantity as the legal criteria now relates to mixing bowl records. However, many B2B Codes of Practice retain the historical legal tolerances. There are legal limits for the percentage of fat and connective tissue that can be classed as ‘meat’ which need expert interpretation to calculate and understand [2]. Nutritionals are calculated ‘as cooked’. If the pack has instructions for both grilling and frying, which one should the laboratory use, as each cooking method will give a different result? What cooking time should they reference, i.e. the minimum or maximum time in the recommended cooking instructions? If frying, should they fry in vegetable oil, and then add the oil to the nutritional values? There are different definitions of fibre, so which one should the laboratory test against? For a ‘low salt’ claim, the salt needs to be 30% lower than the standard product. How do you define a standard product if you are only testing one sample? Is salt measured as sodium or as chloride? If potassium chloride (KCl) is used as a replacement, to give a ‘low salt’ product, then it would skew the chloride result. The protein calculation is dependent on published nitrogen factors. The derivation of these can be unclear and based on relatively few studies. There is a dependence on the cut of meat, breed and feeding regime of the pig. It is accepted to use an average factor for pork, but this may not fairly reflect your product. There are different ways of expressing results [2]: Apparent Fat-Free-Meat Content; Apparent Total Meat Content; Apparent EC Meat Content. |
2. Foreign species DNA: chicken DNA in a beef ready meal
|
What are the compliance criteria, and what does it mean in terms of your product? Many B2B specifications use the Food Standard Agency (FSA) 1% carry-over criteria, but this refers to 1% of the measured DNA relative to the entire extracted mammalian DNA. This cannot be related back to the amount (% w/w) of different meat species in the product. Is there an innocent explanation for the presence of chicken DNA, for example, egg used as an ingredient in the sauce? A judgement call needs to be made. Does it look like potential adulteration, or does it look like cross-contamination? This is a difficult call to make, given the lack of information on % (w/w) of chicken meat in the beef meat component of the meal. In practice this type of result, i.e. a low reading of chicken DNA against a high reading of beef, would only be used to trigger an investigation, rather than take a compliance decision. What other ingredients or species are handled on-site. What is the cleaning process and cleaning verification? |
3. Acrylamide in oven chips
|
Acrylamide must be tested ‘as consumed’. What are the cooking instructions and how should the laboratory account for the likely variation in domestic oven performance? What if the instructions generalise and give words to the effect of ‘leave until golden brown’, rather than state a specific cooking time? If the laboratory uses LC-MS as a test method, then there is likely to be a high level of quantitative uncertainty due to ‘matrix effects’. Has the laboratory tested chips before; were the calibration standards matrix-matched against chips; have they used an isotopic internal standard? What are your compliance criteria, if any? The EC legislation sets benchmark maxima, but these are not compliance criteria. They are based on 85th centile of distribution curves, so statistically 7.5% of products will be above the benchmarks, even if the curves were representative of all types of products on the market - which they are not. The legal framework is to use an ‘as low as reasonably achievable’ (ALARA) approach to your own product, and take all reasonable mitigation steps [4,5], but many B2B Codes of Practice have fixed limits. Many laboratories, if not told otherwise, will report compliance or non-compliance against the EC benchmark levels. |
4. Mineral oils in granola
|
It is difficult to diagnose the source of mineral oil contaminants. Your specification may assume that they are gaseous migrants from recycled board packaging, either the consumer (primary) pack, the store-ready (secondary) packaging, or even the transport (tertiary) carton, or corrugated case. However, there are many other contamination sources in the food chain, e.g. dried raisins are a likely source, either from smoke arising from the drying process, or from oil-treated wooden boxes used for transportation. What are your compliance criteria? There is nothing in the UK but there are legal limits in Belgium and Germany. Some B2B specifications adopt these but are highly contentious. Different regulators segment the many mineral oils into different toxicological categories, with a sum total maximum for each. The test method has huge uncertainty in both identification and quantitation. It uses the sum of all GC peaks, within a retention window, quantified against a surrogate. What can you do with the result? What action can you practically take? For example, you could add a gas-impermeable plastic liner to a breakfast cereal box, but would that be acceptable to consumers? If there is no action that you could take, then the laboratory needs to understand that there is no verdict to be made and that the test result is purely for information. |
5. Trace levels of egg allergen in prawn crackers
|
Allergic reactions are caused by individual proteins and food is a complex mix of different proteins. Which test kit was used? Which antibody? Which protein is it detecting and is it the actual one responsible for the allergenic effect? Does the kit have the same cross-reactivity to cooked egg as to uncooked egg? Has the identification been repeated by a different test kit using a different antibody? Are there any know interferents or cross-reactants? What form is the egg ingredient in? Is it so highly processed that any allergenic effect would be negated? Should you confirm the presence of egg by a DNA (PCR) test? Would the DNA be intact? Just because DNA is present it does not imply allergenicity, but it is supporting evidence. How do you relate the amount of protein present back to the amount (w/w) of egg in the sample? What are your compliance criteria? Zero tolerance is often impractical. Is it appropriate to use the VITAL2 labelling criteria as compliance limits? Is there already any ‘may contain’ labelling on the packet? You have an urgent decision to make. Should you: quarantine stock (awaiting more test results), destroy stock, or go to a full public recall? However good the analysis, analytical results are unlikely to give you as much certainty as you would like to have before making this decision. You will have to make a judgement call. Scenarios should be pre-agreed in advance of the testing, in order to speed the decision, once the analytical result is received. |
Analytical testing is not, in itself, a quality control (QC) procedure. It is quality assurance (QA) so serves as an occasional check that your QC systems are effective. It, therefore, follows that the decisions and actions following a ‘non-compliant’ analytical result should not just be the rejection of a batch code, but it should trigger a challenge of QC systems, and an investigation into why they failed.
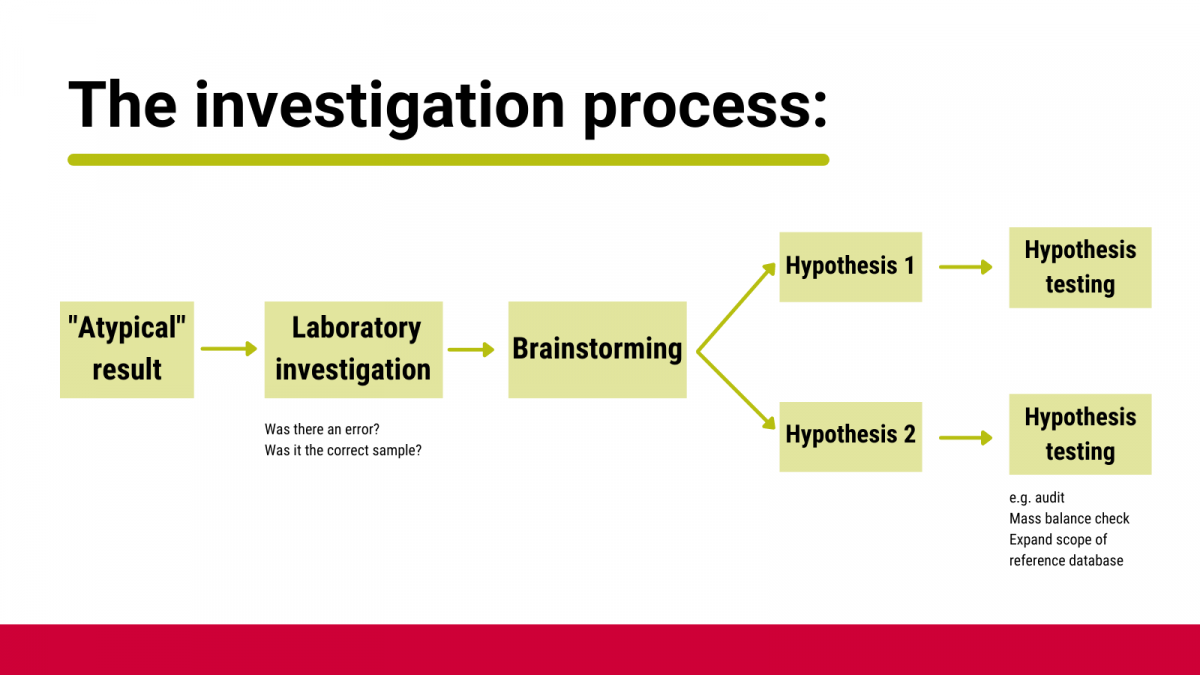
Many modern analytical results are more nuanced than a simple ‘pass’ or ‘fail’. To allow for this, you should have a pre-defined process (Figure 2) as to what to do with ‘suspicious’ results and must NOT involve retesting until you get the result you expect.
Figure 2: Investigation process
- ISO/IEC 17025 Testing and Calibration Laboratories
- ILAC G8 2019 Guidelines on Decision Rules and Statement of Conformity
- Meat and meat products - the calculation of meat content, added water and connective tissue from analytical data 2nd ed. 2007 (Campden BRI)
- FDE Acrylamide Toolbox
- IFST Information Statement ‘Acrylamide in foods’
Institute of Food Science & Technology has authorised the publication of the following Information Statement on How to Interpret Laboratory Results.
This updated Information Statement has been prepared by John Points MIFST, peer-reviewed by professional members of IFST and approved by the IFST Scientific Committee.
This information statement is dated June 2021.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
