June 2021
There are many reasons to test food for chemicals or chemical composition. These include monitoring for undesirable residues, contaminants or by-products, testing for desirable nutrients or flavour compounds, checking recipes or additive composition in the finished product, verification of label claims, checking the authenticity of ingredients, and investigating taints or customer complaints.
The selection of an appropriate test is interlinked with decisions about where in the supply chain to sample, how frequently to sample, and how to target that sampling. This is covered in IFST Information Statement ‘Sampling for Food Analysis - key considerations’. The key principle is that chemical testing is usually a periodic spot check that quality control systems are working as intended. It is not, in itself, a quality control check or a process control point.
Chemical testing |
|
| Applications | Examples |
|
Label verification
|
Nutritional content, fortified vitamins, Quantitative Ingredient Declaration (QUID)
|
|
Chemical residues
|
Pesticides, veterinary medicines, biocides
|
| Natural contaminants | Shellfish toxins, mycotoxins, glycosides |
| Environmental contaminants | Heavy metals, dioxins, PCBs (polychlorinated biphenyls) |
| Process contaminants | Acrylamide, glycidyl esters, MCPD (monochloropropanediol) |
| Packaging migration | Printing inks, mineral oils, phthalates |
| New Product Development (NPD) – health attributes | Phytosterols, functional food chemicals, Omega-3 |
| NPD – quality attributes | Flavour profile, high-potency taste chemicals |
| Competitor product analysis | Functional or technical additives |
| Cross-contamination control | Allergens, meat species, ATP (adenosine triphosphate) swabs |
| Shelf-life studies | Fatty acid composition, rancidity/oxidation |
| Quality control attributes | Hydroxymethylfurfural (HMF) in honey, pH of sauces |
| Authenticity verification | Country of origin, species, variety or grade |
| Adulteration | Melamine, Sudan dyes |
| Customer complaints | Taints and off-flavours |
| Environmental/site monitoring | Factory emissions, process water quality |
This IFST Information Statement is intended to give food industry professionals a background knowledge of different laboratory service offerings and terminology, to assist them in commissioning testing work and in interpreting results. It does not attempt to explain the operating principle of different test techniques, but rather where they are best applied. It is one of a set of IFST Information Statements including ‘How to Choose & Instruct a Laboratory for Chemical Food Analysis’, ‘How to Interpret Laboratory Results’, ‘Microbiological Analysis - key considerations’, ‘Physical Analysis - key considerations’.
|
Terminology In this information statement, we draw a distinction between an analytical technique (i.e. the equipment used to separate, identify and/or measure something) and the analytical method (how that technique is combined with sample preparation and clean-up, and how it is calibrated or configured to give an overall analytical process and result) |
There are a number of ways of classifying methods. The same analytical technique will fall into different classifications, depending upon how it was validated, configured and operated for a specific application. A Venn diagram of different classifications would be far too complex to draw. This makes it vital that a laboratory appreciates the context of their testing requirements, so that they can select not only an appropriate technique but also an appropriate configuration to give a method that is fit for purpose.
For any given test parameter [the analyte(s), component or attribute of the food being measured], there are likely to be a wide variety of different test methods or approaches.
Three ways that methods are frequently classified by laboratories are by:
- the validation approach - standard methods vs. minimum performance characteristics
- whether they quantify the result - quantitative vs. qualitative
- the degree of confidence in ‘positive’ results - screening vs. confirmatory
Expanding on these classifications:
- Validation approach
There are two approaches to ensuring that a test method is fit for purpose.
- In some countries (notably United States and China) ‘standard methods’ tend to be prescribed when testing compliance against legal limits
- In other areas (notably EU), method performance characteristics are specified. Laboratories are free to choose, or develop, the most appropriate method for their own circumstances, but it must meet minimum performance characteristics, which are prescribed in international guidelines or, occasionally, in law.
Both approaches are equally valid. Standard methods are, in principle, more robust, but they take years to validate and approve, and can be superseded by new technology by the time they are published.
- Quantification
Analytical techniques can be configured either to identify the presence of a substance, to quantify the amount present, or to do both. Some techniques are inherently incapable of quantifying the amount of a substance; these are termed qualitative methods. Examples range from spectroscopic techniques (e.g. NMR, FT-IR), and advanced genetic sequencing, to simple colour-change tests. Most techniques, however, are capable of providing some degree of quantification. Whether this provides the quantitative certainty to measure against a limit, or specification, depends on both how the technique is configured, for example the calibration protocol, and on the precision of the specification. Thus, most techniques can be operated as either qualitative or quantitative methods.
- Certainty in a ‘positive’ result
A method which can identify, or measure, a parameter against a specification with sufficient statistical certainty is classed a confirmatory method. However, it is often cost effective to operate techniques in a simpler manner, so that the method still gives confidence in the ‘negative’ results, but positive results are only putative. These are classed as ‘screening’ methods. Customers often instruct their laboratories to retest any putative out of specification samples using a confirmatory method. Some techniques [e.g. enzyme-linked immunosorbent assay (ELISA) and dipsticks] are inherently incapable of confirmatory use, but most techniques can be configured, as either screening or confirmatory methods.
The different inherent uncertainty in the various methods has an important impact on how results are reported against a limit or specification. This is covered in IFST Information Statement ‘How to Choose & Instruct a Laboratory for Chemical Food Analysis’
|
What if there is no confirmatory method for my test requirement? This is notably the case with allergens, where test methods are based on antibody-antigen reactions, but also niche, with new requirements, such as measuring mineral oil packaging migration against quantitative limits. In these cases, laboratories can retest using a second screening method, or retest with a changed test configuration. For example, with a different gas chromatography (GC) column, or different ELISA antibody. Sometimes the combined results, from multiple different screening tests, can give the required confirmatory certainty. |
There are methods that do not fit into this screening versus confirmatory categorisation. These approaches, mainly used for food authenticity testing, are where a pattern from the sample is compared with a database of patterns that are considered ‘authentic’. This gives a probabilistic match. In most cases, the ‘match probability’ quoted by a laboratory should not be equated to the percentage of adulteration i.e. a 95% match does not mean there is 5% of an adulterant present. As well as considering the probability that the sample matches the database, there is also an uncertainty in whether the database truly represents the entire scope of what is considered ‘authentic’. This is covered in the IFST Information Statement ‘Food authenticity testing part 2: Analytical techniques’
No test method has universal applicability to every potential sample type. A test method that works for beef will not necessarily work for a beef pie. The laboratory must verify their method works for each sample type they encounter and may need to tailor it for individual sample types. This means that it is not always possible for a laboratory to consolidate different samples into a single analytical run. Each individual sample-type may need a bespoke, tailored method.
It is important that each analytical run (a batch of samples tested at the same time) includes appropriate control samples. In general, fewer control samples are required for screening methods than for confirmatory methods. Irrespective of whether screening or confirmatory though, the control samples, the calibration standards, and the associated method validation, must be specifically tailored to the sample type.
A minimum number of these tailored quality controls are needed every time the analytical method is run, irrespective of the number of customer samples. This makes it uneconomic for laboratories to test a single sample. They generally schedule many samples into one analytical run. This can either be by holding samples until a ‘batch’ can be tested together, or by processing samples as a continual flow, and interspersing control samples (that are representative, rather than bespoke to each sample type), at predefined frequencies. This latter approach is only appropriate for screening methods, and only for laboratories with a steady high throughput.
It is this scheduling, rather than the hands-on time taken to complete an analysis, generally only a few hours to two days (depending on the method), which is the limiting factor on laboratory turnaround time. There is always a trade-off between cost and turnaround time, with longer turnarounds enabling more economic scheduling.
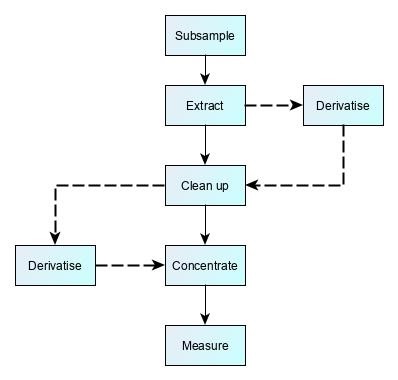
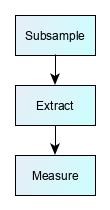
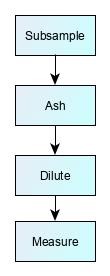
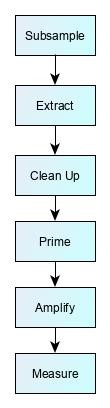
Many test methods require a number of preparatory steps before the measurement technique. This can take anything from a few hours to two or three days. There is often a trade-off in designing these steps. After sample extraction, laboratories will often use sample ‘clean-up step’ to produce a ‘purer’ sample aliquot, i.e. one that contains the target chemical of interest, but with reduced amounts of other chemicals originating from the test sample. This purification process improves method reliability by making it easier for the analytical instrument to make a measurement. However, more steps also mean more scope for losses, more unpredictable recovery, and a weaker measurement signal.
There are almost as many permutations of preparatory steps as there are methods, but some examples are shown below:
|
Chromatographic methods for trace-level organic molecules |
Screening chromatographic methods for trace-level organic molecules |
Atomic adsorption, optical emission, or ICP-MS methods for trace metals |
RT-PCR methods for DNA |
|
|
|
|
|
For highly routine methods, such as Group 1 and 2 nutritionals, automated equipment is available that includes some of the sample preparation and clean up online with the measurement instrument. This brings the unit test price down, but financial payback is dependent on high throughput - thousands of samples per year. This has driven the consolidation of routine testing laboratories into just a few large players.
Analytical techniques used in food testing are based on one of four detection principles:
- biological techniques - detect a cellular response to the analyte e.g. inhibition of bacterial growth
- biochemical techniques - based upon the specificity of a biochemical mechanism e.g. pairing of DNA strands or antibody-antigen pairings
- physicochemical techniques - detect a physical characteristic of a chemical e.g. wavelength of light absorbed or emitted or molecular mass
- physical techniques - simple measurements such as weighing, or more complex techniques, such as particle size measurement. These are covered in IFST Information Statement ’Physical Analysis - key considerations’
This list is not intended to be exhaustive but includes some of the methods most commonly used in food analysis.
- Biological techniques
- Bacterial growth inhibition assays
|
Example |
DelvoTest®SP - antibiotic residue screening test used in dairy industry |
|
Quantitative? |
No; qualitative only |
|
Confirmatory? |
No; screening only |
|
Turnaround |
2 to 3 hours, if on-site |
|
Capital cost |
None |
|
Cost per test |
Very cheap |
|
Strengths |
Portable; does not need specialist operators or equipment |
|
Weaknesses |
Identifies if an antibiotic residue is present, but not which antibiotic |
|
Watch outs |
To detect a wide range of antibiotics at their legal limit, it must be oversensitive, i.e. reports ‘positive’ at well below the legal limit, for some. Will not detect all antibiotics. |
2. Biochemical techniques
a. Lateral flow assays
|
Example |
‘Dipstick’ tests for allergen cleaning verification |
|
Quantitative? |
No; qualitative only |
|
Confirmatory? |
No; screening only |
|
Turnaround |
A few minutes, if on-site |
|
Capital cost |
None |
|
Cost per test |
Cheap; up to £20+ for the most expensive test kits |
|
Strengths |
Portable; does not need a specialist operator |
|
Weaknesses |
Positive results always need to be sent to a laboratory for confirmation |
|
Watch outs |
The way that the swab sample is taken, affects the validity of the result, more than the operation of the test itself |
- ELISA (enzyme-linked immunosorbent assay)
|
Example |
Commercial plate tests for allergens in food |
|
Quantitative? |
Can be operated qualitative or quantitative |
|
Confirmatory? |
Identification is not definitive; subject to false positives |
|
Turnaround |
1 to 5 days if sent to laboratory |
|
Capital cost |
Low |
|
Cost per test |
Cheap |
|
Strengths |
Easy and quick to operate; low entry barrier for laboratories |
|
Weaknesses |
Lack of definitive confirmation; dependency on operator skill (accurate pipetting) |
|
Watch outs |
Kits being used beyond their validated scope, e.g. different sample types; unchallenging ‘black box’ acceptance of kit-generated results, without assessment of the performance characteristics on the day |
- RT-PCR (polymerase chain reaction)
|
Example |
Detection of undeclared meat species in a processed food |
|
Quantitative? |
No; DNA can be semi-quantified, but does not relate back to meat quantity |
|
Confirmatory? |
Yes |
|
Turnaround |
1 to 5 days, if sent to laboratory |
|
Capital cost |
Moderate |
|
Cost per test |
Moderate |
|
Strengths |
Can be highly sensitive; good confidence in confirmation of identity |
|
Weaknesses |
Can be oversensitive |
|
Watch outs |
Oversensitivity in some cases, and difficult to draw trends in (semi-) quantification, particularly when more than one species ingredient is in the same product. In other cases, a ‘non-detect’ may be because the DNA is no longer intact, for example in pâté, stock, or slow-ripened cheese |
- Next Generation Sequencing (NGS)/Whole Genome Sequencing
|
Example |
DNA identification of species of white frozen block fish ingredient |
|
Quantitative? |
No |
|
Confirmatory? |
Yes |
|
Turnaround |
3 to 10 days, if sent to laboratory |
|
Capital cost |
High |
|
Cost per test |
High |
|
Strengths |
Definitive results for single ingredients, or simple mixes |
|
Weaknesses |
Inapplicable to more complex mixtures. As a rough rule, inapplicable if the ingredient of interest is < 10% of the mix |
|
Watch outs |
Identification is taken from public databases. Need to be careful with species nomenclature, i.e. use Latin names to avoid confusion). Databases are constructed from species specimens collected and stored over time, and sometimes the taxonomical identifications were unchecked, or have been revised since the specimen was collected. |
3. Physicochemical techniques
- Low Resolution NMR (nuclear magnetic resonance)
|
Example |
Online Foss machine in meat plants; fat nutritional testing |
|
Quantitative? |
Approximately |
|
Confirmatory? |
No |
|
Turnaround |
Real-time; online measurement |
|
Capital cost |
Moderate |
|
Cost per test |
Low |
|
Strengths |
Cheap to operate; push button operation |
|
Weaknesses |
Crude and approximate measurement |
|
Watch outs |
Overreliance on results accuracy |
- High Resolution NMR
|
Example |
AIJN SNIF-NMR database |
|
Quantitative? |
No |
|
Confirmatory? |
Gives a probability fit against an ‘authentic’ database; can be used in other modes, to confirm identity of individual sugars |
|
Turnaround |
2 to 10 days |
|
Capital cost |
High |
|
Cost per test |
Medium |
|
Strengths |
Capable of untargeted analysis |
|
Weaknesses |
Reliant on appropriateness of the reference database |
|
Watch outs |
Need to ensure transparency, that the reference database is appropriate, and that it includes production systems and ingredient types that are representative of one’s own sample |
- GC-FID [gas Chromatography with flame ionisation detection]
|
Example |
Mineral oils packaging migration; fatty acid profiling |
|
Quantitative? |
Yes |
|
Confirmatory? |
Provisional, not definitive |
|
Turnaround |
2 to 10 days |
|
Capital cost |
Moderate |
|
Cost per test |
Medium |
|
Strengths |
Universal detector response to any hydrocarbon compounds; can extrapolate response from one compound to another. GC is good at separating complex component mixtures. Very robust |
|
Weaknesses |
Not as sensitive as some other GC detectors. Subject to interferents, hence difficulties in measuring low-level contaminants in complex samples. Non-volatile analytes, e.g. fats, need derivatisation, adding to method complexity |
|
Watch outs |
Need to check if the analyte was quantified against a known reference standard, or against a surrogate, and if compounds were measured individually, or summed |
- GC-MS (gas chromatography with mass spectrometric detection)
|
Example |
Flavour profiling |
|
Quantitative? |
Yes |
|
Confirmatory? |
Yes |
|
Turnaround |
2 to 10 days |
|
Capital cost |
Moderate |
|
Cost per test |
Moderate |
|
Strengths |
Flexible, wide range of application, sensitive. GC is good at separating complex multi-component mixtures |
|
Weaknesses |
GC is not applicable to non-volatile, or highly polar compounds, unless the method includes a derivatisation step. Instrument can become fouled by dirty samples, losing sensitivity |
|
Watch outs |
Need to check the masses measured are diagnostic of the analyte, e.g. if a derivative is used, the laboratory is not measuring a mass fragment from the derivatising agent. Also, whether the method has been validated using a pure chemical standard |
- LC-MS (liquid chromatography with mass spectrometric detection)
|
Example |
Pesticide residues, acrylamide, Sudan dyes, Melamine |
|
Quantitative? |
Yes |
|
Confirmatory? |
Yes |
|
Turnaround |
2 to 10 days |
|
Capital cost |
Moderate/high |
|
Cost per test |
Moderate |
|
Strengths |
Robust, near-universal range of applicability, sensitive, selective |
|
Weaknesses |
Susceptible to ‘matrix effects’. Detector response varies from one sample extract to another so laboratory needs to mitigate this. Strongly ionic analytes. salts and sugars, need chromatography conditions that risk fouling the detector |
|
Watch outs |
Differentiating between substances of the same mass, e.g. chemical isomers, where one isomer is active/toxic, and the other is not. Need to check if the method been validated using a pure chemical standard |
- LC-UV/Fluorescence
|
Example |
Vitamins |
|
Quantitative? |
Yes |
|
Confirmatory? |
Provisional, not definitive |
|
Turnaround |
2 to 10 days |
|
Capital cost |
Moderate |
|
Cost per test |
Medium |
|
Strengths |
Wide applicability, robust, low entry barrier for laboratories |
|
Weaknesses |
Susceptible to interference (false positives). Many fluorescent methods need derivatisation, adding to the method complexity |
|
Watch outs |
Susceptibility to interference is highly dependent on wavelength. Laboratory reports often do not reveal if conditions were highly selective (e.g. UV 450nm) or poorly selective (e.g. UV 210nm) Check measurement units (e.g. IU vs mg) and nomenclature used by laboratory. This may differ from supplier information, e.g. all-rac-α-tocopheryl acetate, d-α-tocopherol and d-α-tocopherol acetate are all used for vitamin E but have different IU activities |
- Ion Chromatography with Electrochemical Detection
|
Example |
Sugars |
|
Quantitative? |
Yes |
|
Confirmatory? |
Provisional, not definitive |
|
Turnaround |
2 to 10 days |
|
Capital cost |
Moderate |
|
Cost per test |
Medium |
|
Strengths |
The only option, for some types of analytes, particularly salts and sugars, for a detector that is both responsive and will not be fouled by the conditions needed |
|
Weaknesses |
Niche applicability |
|
Watch outs |
Correct identification of structural isomers and similar compounds |
- ICP-MS (mass spectrometry with inductively coupled plasma ionisation)
|
Example |
Trace contaminants - heavy metals |
|
Quantitative? |
Yes; at low concentrations only |
|
Confirmatory? |
Yes |
|
Turnaround |
2 to 10 days |
|
Capital cost |
High |
|
Cost per test |
Medium |
|
Strengths |
Very sensitive and selective for metals and minerals |
|
Weaknesses |
Not quantitative at higher concentrations. Only applicable to metals and minerals |
|
Watch outs |
Known interferents; list from complex ions, formed from plasma gas e.g. argon complexes. May result in poor quantitation. |
- ICP-OES (optical emission spectroscopy with inductively coupled plasma excitation)
|
Example |
Micronutrients, metals |
|
Quantitative? |
Yes |
|
Confirmatory? |
Yes |
|
Turnaround |
2 to 10 days |
|
Capital cost |
Moderate |
|
Cost per test |
Medium |
|
Strengths |
Applicable to higher concentrations of metals and minerals than ICP-MS |
|
Weaknesses |
Not as sensitive at lower concentrations as ICP-MS |
|
Watch outs |
Possible matrix effects on quantitation |
4. Physical techniques
a. Weighing
|
Example |
Drained weight of glazed frozen food |
|
Quantitative? |
Yes |
|
Confirmatory? |
Yes |
|
Turnaround |
1 to 2 days |
|
Capital cost |
None |
|
Cost per test |
Cheap |
|
Strengths |
Simple |
|
Weaknesses |
Need standardised method protocols for comparability of results e.g. standardised ratio of sample size to sieve size and mesh |
|
Watch outs |
Sampling protocols are critical. See IFST Information Statement ‘Sampling for Food Analysis - key considerations’ |
b. Ashing then weighing
|
Example |
Dietary fibre |
|
Quantitative? |
Yes |
|
Confirmatory? |
Identification is by calculation and inference |
|
Turnaround |
1 to 5 days |
|
Capital cost |
None |
|
Cost per test |
Moderate |
|
Strengths |
Simple; low entry barrier for laboratories |
|
Weaknesses |
Calculations are underpinned by assumptions that may not always be correct |
|
Watch outs |
Results must be expressed ‘as measured by a particular method’. If not, results from different assays, or specifications, are not comparable. See IFST Information Statement ‘Dietary Fibre’ |
Confirmatory methods: Give the required confidence that ‘positive’ or ‘non-compliant’ results are correct
Performance characteristics: Metrics by which a method’s suitability can be specified and quantified. Examples include specificity, selectivity, working range, accuracy, precision, ruggedness, stability and uncertainty. See IFST Information Statement ‘How to Choose & Instruct a Laboratory for Chemical Food Analysis’
Qualitative methods: Identify the presence or absence of the parameter of interest, but do not measure the amount
Quantitative methods: Measure the amount of the parameter of interest that is present
Screening methods: Give a confident result that the parameter of interest is not present or is below the specification limit i.e. that the result is ‘negative’ at a known confidence level. There is insufficient confidence that putative ‘positive’ results are correct
Standard methods: Detailed procedures which have been validated by comparative tests in different laboratories. The detailed procedure is then published by an authoritative body and/or cross-referenced in national legislation
Validation: Proving, by experiment, that the method is fit for its intended purpose.
Institute of Food Science & Technology has authorised the publication of the following Information Statement on Chemical Analysis - key considerations.
This updated Information Statement has been prepared by John Points MIFST, peer-reviewed by professional members of IFST and approved by the IFST Scientific Committee.
This information statement is dated June 2021.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.