
June 2021
Selection of the right laboratory and the appropriate testing suite are key considerations for anyone who requires microbiological testing of samples, whether the tests are carried out internally, or by a third-party laboratory. A laboratory may not have the facility to test some pathogens, for example, Escherichia coli O157.
External laboratories selected for analysis should conform to a recognised certification scheme, which complies with ISO 17025 or similar. It is worth asking what positive and negative microbial control strains are used, because some may not be appropriate for the microbial flora in the ingredients or finished products. Also, some of the tests that are required may not be in the scope of accreditation of the laboratory, so it is important to consider how these tests will be conducted and reported, and what quality system is used for those analyses.
A glossary of acronyms, terms and standard microbiological expressions is contained at the end of this document. There are a number of sources of more detailed information for sampling and testing methods in general, and for particular food commodities which can be found in the reference list. A key reference used to compile this IFST Information Statement is the second edition of the IFST Handbook of Microbiological Criteria for Foods, 2nd edition.
You might have a critical raw ingredient that undergoes minimal, or no, processing where a key food safety, or food spoilage microbe, must be present at less than a certain level. It could be a legal requirement, as part of EU Regulation 2073/2005 on microbiological criteria for foodstuffs. It could be part of a finished product verification criteria, or for the protection of sensitive products and ingredients, or a complaints investigation into a quality or taint defect.
Microbiological results can also be very useful in trend analysis, so that any drift or movement towards an unsafe product, or one where the shelf life may be reduced, can be detected and acted upon, before it is too late.
A risk-based approach should be taken to determine the location and timing for taking samples, considering where contamination is most likely to occur, or at what stage of the process. A positive release approach (pathogen absent) for finished products is not recommended, because there is always a level of uncertainty in any result.
It is very important that samples for microbiological testing are not contaminated during the sampling procedure, hence the process must be conducted aseptically. This means that the aliquot for microbiological testing must be taken first, if a range of analyses are to be conducted on any sample. Samples then need to be kept in the same state, before testing, i.e. frozen remains frozen and chilled remains chilled. Do not freeze a chilled sample, unless there will be a considerable time lag between sampling and testing, since the results may not be representative of a sample taken from a production line. Ideally, chilled samples should be analysed within four hours of sampling.
The location, type, time and date, relating to a sample, must all be clearly recorded and logged. The laboratory will assign its own laboratory codes which must be correlated with the internal sample identity.
If a sample is to be used in a cross-check on a Certificate of Conformance/Analysis, where possible, the same testing methodology should be used, or the differences between the methods understood, in order to be able to compare ‘like with like’.
More general details on sampling can be found in the IFST Information Statement ‘Sampling for Food Analysis - key considerations’.
1. Environmental monitoring
Environmental monitoring can be part of the ongoing suite of tests, or in response to a contamination incident, for example, if Salmonella or Listeria were detected in the final product. This may require a systematic approach to locating the source, by starting with drain sampling, then focusing down on surfaces, or ingredients, once an area is pinpointed.
If routine, then walls, doors or strip curtain divides, working surfaces and key areas of machinery should be included. Tests are usually for background flora (see ‘Spoilage organisms’ below), or indicator (or index) organisms that indicate that pathogens may be present (e.g. Listeria spp. for Listeria monocytogenes; Enterobacteriaceae or faecal coliforms for Salmonella).
Rapid methods for environmental contamination include those using a luminometer to detect Adenosine Triphosphate (ATP). These can be used for surfaces or post clean-in-place (CIP) wash water and give results in 10 to 20 seconds. It is worth noting that at least 104 cfu bacteria are required to trigger a minimal response, with food residues and fruit juice cells, giving a greater response. ATP testing should therefore be seen as a cleaning verification tool, not to determine microbial cleanliness, however, it is a very useful rapid method. Other rapid methods include colorimetric techniques used on liquid processing lines, flow cytometry and DNA/RNA based techniques.
In all cases, baseline levels need to be determined so that upwards trends, or spikes, can be identified and dealt with. It may be that some surfaces or machinery do not give results below the limit of detection, due to their age or cleanability, but this may not contribute to excessive product contamination.
2. Product testing
- Spoilage organisms
Before requesting a test suite, it is important to understand the following:
- capability of the test to recover microorganisms
- when the results are required
- sensitivity and the cost of the method.
Dilution of the sample is usually required to be able to enumerate the microflora of a sample. It is helpful to have an approximate idea of the contamination level of a sample, or the laboratory might not be able to enumerate either very low or very high levels of contamination. All methods have a lower limit of detection; a negative result may not mean a microbe is absent which could be significant with respect to pathogens (see below). Dilutions range from 10-1, for lightly contaminated foods, to as low as 10-6, for heavily contaminated. When selecting the dilution series, it is important to ensure that pathogens present at low levels, are not missed by using only the more dilute aliquots, for the more common organisms.
The test method chosen will define the microorganisms detected and the count. For bacteria, an Aerobic Colony Count [ACC, also referred to as a Total Viable Count (TVC) or Aerobic Plate Count (APC)] can be conducted using Plate Count Agar, at temperatures ranging from 20 to 37°C, for 48 to 120 hours – see Table 1. Tests to isolate more specialised microorganisms can be carried out at temperatures above or below.
High ACC’s can be expected on unwashed salads and vegetables, and from fermented foods, and therefore testing for this parameter is not useful. This applies to any food or ingredient where high natural levels can be expected.
To determine water quality and safety, a Heterotrophic Plate Count (Total Heterotrophs) is often used, with a two plate two temperature method - see Table 1. The temperature selected is dependent upon the type of sample, its typical temperature and in some cases the country of origin of the method used. A direct comparison of results between methods should therefore not be made and the resultant flora isolated can vary considerably. Table 1 outlines a selection of some of the rationales for ACCs and HPCs.
Table 1: Aerobic colony count protocols for bacteria
|
Method |
Incubation Temperature (°C) |
Incubation Time (hours) |
|
American Public Health Association |
20 32 or 35 |
72-120 48 |
|
ISO – Total Heterotrophs for water |
22 36 or 37 |
72 48 |
|
ISO/BS/EN for food and feed |
30 |
72 |
|
Association of Official Analytical Chemists |
35 |
48 |
|
Brazilian Ministry of Agriculture |
36 |
48 |
Whether a food is heat processed, with a kill step during production, or processed without heat, will affect the final flora of the product, and determine acceptable microbial limits, and the types of microorganisms for which to analyse.
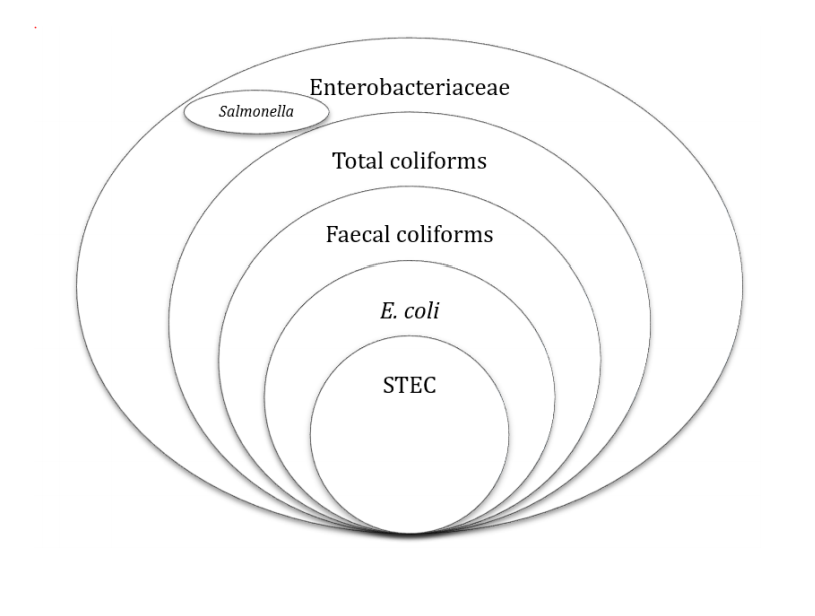
Indicator organisms highlight the efficacy of a process, its hygiene status and potential faecal contamination. Index organisms mark the potential presence of a pathogen. Confusingly, the term indicator organism has also been used, by some, for potential presence of a pathogen. Enterobacteriaceae and coliforms are used as indicators of hygiene and process efficacy for pasteurisation, E coli or faecal coliforms as a faecal indicator, and non-pathogenic spores for the efficacy of a high temperature process. Each microbial group has its merits and demerits. Figure 1 shows the relationship between the different groups.
Figure 1: Relationship between Enterobacteriaceae, coliforms, faecal coliforms, Salmonella, E. coli and STEC
Yeast and mould counts can be used as general hygiene indicators, and also in spoilage investigations in key industries, for example soft drinks, confectionery, bakery, and dried foods. Plates are typically incubated at 25°C for five days, however problems can arise with the interpretation of yeast and mould counts in spoilage investigations. There are a number of methods, with different media, temperatures, and each method may isolate a different mould flora, which may not be related to the mould or group of moulds causing spoilage.
Lactic acid bacteria (LAB) are present at high levels in most fermented foods, and therefore their analysis in this type of food is not recommended. They are also environmental bacteria and can be expected in unwashed salads and other vegetables. They can indicate process failure or recontamination after processing, or cleaning failures, in the soft drinks industry, where they can cause considerable spoilage.
Tests for aerobic and anaerobic bacterial spores are often used to indicate process failure. In these cases, it is important to know the thermal process used, because each species’ spores have unique thermal properties. These are subdivided into tests for mesophilic and thermophilic spores. Survival of spores may not indicate process failure, if the product formulation has other control factors, to inhibit surviving spores.
Each test has a minimum turnaround time that is fixed, for example, 5 days for yeasts and moulds; LAB 2 days at 35°C, 3 days at 25°C; aerobic spore counts at 7°C for 10 days, 30 -35°C for 2 days, or 55°C for 2 days. Sometimes these can be extended if the process, or product, includes a sub-lethal thermal process, or other intrinsic control factors which inhibit recovery of viable cells. This is usually outside the scope of the test but can be helpful in investigations.
Coliforms, Enterobacteriaceae, LAB, yeasts and moulds are commonly found on fresh produce, therefore tests for their presence are of limited use. The presence of faecal coliforms or E. coli could indicate faecal contamination of salad vegetables, and the potential presence of Salmonella.
The origin of ingredients can affect the microflora, for spoilage bacteria and moulds, and for pathogen serovars.
b. Pathogens
Isolation methods for pathogens fall into two categories: detection (presence/absence) and enumeration.
i. Detection methods
These are used where, either the pathogen is present at low numbers in the particular food, is difficult to detect in the food, in a non-homogenous distribution, or poses a severe hazard to the general population, or consumer group, for which the food is intended. There are usually three or four stages, which makes it a lengthy process.
Standard enumeration methods are similar to those for spoilage or environmental organisms, with dilution (often to a lesser extent), because they may not be present at such high levels. The timescales range from 18 - 24 hours to 4 - 5 days, depending upon the organism and the test method. ISO or national standards body methods tend to be slower but are more likely to work with a greater range of isolates.
It is helpful if the laboratory is able to adapt methods if the samples taken are part of an investigation to determine why a pathogen has been found in a food or in the environment. It is important to remember that a test result is presumptive until confirmed.
Rapid methods can be divided into two types: those that provide a more rapid turnaround for the isolation and detection phase, or a rapid confirmation stage. For the former, a non-selective or selective amplification stage is usually required for a rapid isolation method, or it is likely that the small sample aliquot used for the rapid test may not contain any of the relevant pathogen cells. The end result may or may not require further confirmation, depending upon the type of rapid isolation and detection test used.
ii. Confirmation methods
These are either slow [typically more complex ISO or another standards body (so-called ‘Gold Standard’), which usually work with the widest range of isolates of a particular species], or more rapid techniques. If the latter, it is preferable that they have been accredited against the ‘Gold Standard’, either by the laboratory, or more usually by the national standards laboratory, or similar. A rapid confirmation test should give comparable results to the standard method, otherwise false positives or negatives may result.
Rapid confirmation methods include serological (ELISA or similar), miniaturised multi-well tests (API or similar), or DNA/RNA based tests.
Each method has a minimum sensitivity, i.e. a record of ‘absent’ or ‘not detected’ means to the lowest sensitivity of the method. Similarly, ‘Too Numerous To Count’ (TNTC) means that an incorrect dilution series was used for the test, such that common microbes grew too close together to be able to count as individual colonies.
Microbiological results can be very useful in trend analysis, so that any drift or movement towards an unsafe product, or one where the shelf life may be reduced, can be detected and acted upon, before it is too late.
- Relevant physical parameters
It can be helpful to request, or to already have information on, key product parameter tests. They can be used to guide the testing suite and include:
- Water activity (aw) - this can determine which microorganisms are able to grow in the product since each microbe has a very clear aw below which it cannot grow. The theoretical aw may not be the same as the actual value. Xerophilic yeasts and moulds are adapted to growth at extremely low aw and may take some time to grow in the product
- Brix - useful to calculate for juices and concentrates, as like aw it will determine which microorganisms can grow
- pH - of a product can determine which microorganisms can grow and can also influence their heat resistance at any given value. Some pathogens, and many spoilage microorganisms, are able to grow or survive at low pH. Yeasts and moulds are largely unaffected by low pH over the typical ranges of food and drinks.
3. Shelf life and challenge testing
It is important, when designing a shelf life testing protocol, that appropriate time intervals are considered, particularly if the budget is limited. Are changes in microflora (growth or death) expected at the beginning or the end of the trial period? It may be more efficient to have uneven time intervals, focusing on the expected key period, but still retaining time points until the end of the trial, so that potentially useful data is not lost. In addition, the testing suite could vary, from the start to the end of the trial, for the same reason.
Challenge testing is carried out when an important pathogen is present infrequently, but it’s growth or survival in the product would render it unsafe for consumption. The product is therefore ‘challenged‘, with the microorganism of concern, under typical storage conditions. In this case, the use of strains, their adaptation to the test matrix, the inoculation methods, the experimental matrix (factors x timepoints), the interpretation of results, and the likely background flora are all important factors for the laboratory to consider.
4. Testing specific foods
The IFST Handbook of Microbiological Criteria for Foods, 2nd edition gives a comprehensive set of criteria for different food types, with a helpful indexed table cross-referencing the individual criteria tables. For example, if biscuits, breakfast cereals, cakes, chocolate, confectionery and potato crisps are selected, then Table N ‘Dried foods - ready to eat’, is referenced. In other cases, more than one table may be referenced. For example, selecting spices points the reader to Table N, as well as Table M ‘Dried foods - to be cooked’ and Table O ‘Dried heat processed foods - ready to eat after rehydration'.
ATP: Adenosine Triphosphate; chemical marker used as a rapid hygiene test for surfaces and water
Aw: Water activity (see below)
Colony forming unit (CFU): basic microbial unit that can be measured as an individual colony on a plate usually comes from a single cell
Coliform: type of bacteria used as an indicator organism, commonly found on plants and in soil. Coliforms are members of the Enterobacteriaceae and Escherichia coli (E. coli) is a faecal coliform
Confirmation: test used to confirm the initial presumptive result for a pathogen which employs more specific analytical criteria than the initial test
Enterobacteriaceae: family of bacteria that can be used as indicator organisms. Includes coliforms, Salmonella and some other gram-negative pathogens
Extrinsic factor: external factors to the food that affect microbial growth, i.e. temperature, humidity and gas atmosphere
Faecal coliform: an organism, found in faeces, that can be used to indicate contamination of food or water with faeces, and as an index for the presence of faecal pathogens e.g. E. coli
Index organism: signals the increased likelihood of the presence of a pathogen in a sample e.g. E. coli and faecal coliforms
Indicator organism: common microorganisms used as indicators of poor processing, sub- standard hygiene, post-process contamination, low quality raw materials or contaminated water. N.B. The terms Indicator and Index are often incorrectly used interchangeably
Intrinsic factor: aspects of a food that affect microbial growth, e.g. pH, aw, nutrient content, redox potential
Mesophile: microorganism with typical optimal growth temperatures of 28 to 43°C (ranging from 5 to 52°C), depending on the organism
Osmophile: microorganism that prefers to grow in food and drinks with high levels of sugar. Typically used to describe yeasts and moulds that are able to grow in high sugar drinks and concentrates. A type of xerophile (see below).
Presence/absence test: used where presence of a pathogen is usually unacceptable, and absence acceptable. Usually comprises three or four stages: non-selective enrichment; selective enrichment; recognition (usually on agar plates); confirmation. Often carried out when the number of target organisms is very low, the organism is stressed, detection is more important than the level (because of the food type or intended use, for a susceptible consumer), or if there are large numbers of competitor organisms, which could supress detection of the pathogen. See Two-class plan below.
Presumptive result: until a pathogen test result is confirmed, it is described as presumptive (see ‘Confirmation’ above). The initial test method is designed to increase the likelihood that a pathogen is isolated and detected, but this may also detect similar, non-pathogenic bacteria, which need to be excluded to confirm the initial diagnosis
Psychrophile: microorganism that prefers to grow in colder conditions. Their optimum temperature is 15°C, and they cannot grow at 30°C
Psychrotroph: microorganism that can tolerate low temperatures but has an optimum range from 20 to 30°C.
STEC: Shiga-toxin producing E. coli. A virulent subset of E. coli that can cause serious food poisoning, e.g. E. coli O157
Thermophile: organism that prefers to grow at high temperatures, ranging from 30°C to 70°C (optimum 50 - 65°C)
Thermotolerant: can tolerate high temperatures, but also able to grow at lower temperatures. A thermoduric organism can tolerate, but may not be able to grow at, high temperatures e.g. spore-forming organisms
Three-class plan: builds in a degree of acceptance of samples of intermediate microbiological quality, whilst still retaining an upper cut off point. It is based on the following criteria: n = number of samples to be tested (often 5); m = microbiological limit that separates good quality from marginal quality; M = microbiological limit above which sampling results are unacceptable; c = number of units allowed to yield results between m and M (often 2). The number of units allowed to exceed M is usually 0.
Too numerous to count (TNTC): when the types of microorganisms isolated, or the dilution selected, has caused the agar plate to be overrun, such that it is not possible to count the actual number of colonies
Two-class plan: designed to decide on acceptance, or rejection of a sample, based on the following criteria: n = number of samples to be tested; m = acceptance criteria (for a pathogen this is usually ‘absent in x g/ml’); c = number allowed to exceed acceptance criteria (usually 0)
Water activity (Aw): amount of water available in a food for microbial growth. It is usually measured by determining the relative humidity of the air, above a sample of the product in a water activity meter, at a given temperature. The water activity of a sample at the same moisture content varies with temperature. Xerophiles and osmophiles are able to grow at low water activities
Xerophile: yeast or mould, able to grow at a water activity of 0.85, or less, under one set of growth conditions.
FDA Bacteriological Analytical Manual 1998; online version 2021 Bacteriological Analytical Manual (BAM) | FDA
Forsythe, S.J. 2010 The Microbiology of Safe Food, Chichester, Wiley-Blackwell, 2nd edition
ICMSF 1996, Microorganisms in Foods 5: Characteristics of Microbial Pathogens. London: Blackie Academic and Professional
ICMSF 2005, Microorganisms in Foods 6: Microbial Ecology of Food Commodities New York: Kluwer Academic and Plenum Publishers, 2nd edition
ICMSF 2011, Microorganisms in Foods 8: Use of Data for Assessing Process Control and Product Acceptance, Cham, Switzerland, Springer International Publishing
ICMSF 2018, Microorganisms in Foods 7: Microbiological Testing in Food Safety Management, Cham, Switzerland, Springer International Publishing
IFST 2020, Handbook of Microbiological Criteria for Foods, London, IFST, 2nd edition
Roberts D., Greenwood M. 2002, Practical Food Microbiology, Chichester, Wiley-Blackwell, 3rd edition
Stannard, C.J., Petitt, S.B. and Skinner, F.A. 2009, Rapid Microbiological Methods for Foods, Beverages and Pharmaceuticals, Chichester, Wiley-Blackwell.
Institute of Food Science & Technology has authorised the publication of the following Information Statement on Microbiological Analysis - key considerations.
This updated Information Statement has been prepared by Dr Peter Wareing RFoodSM, FIFST, peer-reviewed by professional members of IFST and approved by the IFST Scientific Committee.
This information statement is dated June 2021.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
