February 2023
There is no doubt that food systems must operate to be more efficient. For every joule of energy placed into the food system, we must try to obtain as much energy from it. However, we ultimately depend on an agricultural system that captures solar energy. More efficient processing, manufacturing and retailing are focussed on reducing energy costs, and this is realised when process and material improvement is achieved. Geopolitical events have changed how food is traded globally, and energy supply has become a dominant issue that aligns with sustainable outcomes. The food production system depends on natural gas to produce nitrogenous fertilisers and fuels to cultivate and harvest biomass. Optimising the balance across these systems requires innovation and technologies that improve efficiency and interact positively with consumers and society. The COVID-19 pandemic has had a wide-ranging impact on complex supply chains. Fuel price increases have hit transport costs and have been exacerbated by conflict in the Ukraine, leading to disruption of global wheat and sunflower oil supplies. Home grown agri-food and environmental strategy decisions are being made to address sustainability and climate change questions, which are likely to add further stress to the supply network. We are witnessing changes in land use with a push towards re-wilding and reforestation to balance our carbon emissions. At the same time, there is growing pressure from housing demand on the Green Belt. A move to plant-based diets is seen as a means of reducing greenhouse gas emissions, such as methane production from livestock. However, the push to less intensive agricultural practices means that there needs to be a paradigm shift in agricultural productivity that offsets the reduced inputs and a reduction in productive land area against the continued demand for affordable, nutritious and tasty food.
Many researchers and technologists have focused on genetics and molecular biology to find ways of overcoming the limitations inherent in traditional approaches to breed improvement. This has brought revolutionary improvements in our understanding of plant and animal characteristics that take us far beyond DNA and inheritance. For example, DNA profiling, genomics, transcriptomics, proteomics and metabolomics have produced essential insights, together with recent studies of post-transcriptional modification and epigenetics. These naturally occurring processes can bring about heritable variation expressed as the phenotype, or traits, in organisms. Plant tissue culture techniques have provided an effective tool for propagating large numbers of targets for screening. There are stresses on micro-propagated tissues grown in vitro that may generate genetic variability. This may be due to environmental conditions or culture media composition. Such somaclonal variations arise because of gene mutation or changes in epigenetic markers. Desirable phenotypes or traits can be screened for and harnessed in conventional breeding programmes.
With the advent of rapid genetic testing and diagnostic tools such as PCR, a term familiar to everyone since the beginning of COVID-19, we have seen rapid advances in molecular biology and biotechnology, which have impacted all aspects of the life sciences. We have a greater depth of understanding of both the genetic origins of the produce we eat and of the diseases and environmental changes that jeopardises food production. One thing that has come to light is that crop genetic diversity has been declining rapidly, threatening food security and sustainable development [1].
Traditionally, early selective breeding developments included identifying visible differences between crop plants or livestock, for example shorter cereal stems, higher yielding dairy cows, and actively crossing individuals with those advantageous traits to produce hybrids with those enhanced properties.
It was found that the exposure of reproductive tissues such as pollen, seeds and eggs or sperm to certain chemicals or to radiation could increase the rate of mutations leading to natural variation. This increases the rate of mutation and the range of genes affected. Some parts of the genome are more stable than others, more susceptible to induced mutagenesis, and more likely to conserve changes in the genome from one generation to the next [2]
Screening for anti-nutritional factors is required to ensure the new foods are free of toxins, off flavours or undesirable tastes and that the new desirable traits persist over generations. The number of isolated mutant candidates may be huge, and the traditional selection and backcrossing process can take decades before a commercial breed or variety comes to the market.
There have been concerns for many years regarding the erosion of genetic diversity in crop plants [3]. Commercial varieties can be rendered obsolete as emerging pathogen variants overcome their disease resistance. This can lead to catastrophic global crop failure where there is a dependence on a single cultivar, e.g. the Cavandish cultivar of banana is being threatened by a new variant of Fusarium wilt (TR4). This follows the variant (R1) decimating the earlier dominant banana variety “Gros Michel” in the 1950’s [4]. Resistance to emerging diseases or susceptibility to environmental change may impact crops that are derived from a limited gene pool of wild relatives. Cultivated varieties of potato (Solanum tuberosum) are generally susceptible to late blight disease, which is typically controlled by multiple applications of fungicide. However, there is a great deal of diversity including late blight resistance in the remaining wild relatives of modern cultivars, typically growing wild in South America. However, selecting for multi-gene disease resistance using conventional methods has met with limited success for the prolonged control of blight as the pathogen is adept at overcoming resistance in new cultivars [5]. The key threats to staple foods are a key target for new molecular biology and biotechnology tools and in precision breeding techniques developed to complement conventional approaches. New breeding technologies may hold the key for securing a sustainable future for food supply in a more timely manner than has previously been possible in the face of emerging challenges.
Gene editing (GE) comprises a group of methods and tools that help to make up an array of New Breeding Technologies (NBT). Advances in breeding technologies using molecular biology techniques in the 1990s ultimately led to the development of new tools to develop organisms with desired traits. Some modern mutagenesis tools, which perform site-specific directed and controlled manipulation of genes and includes, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and Clustered Regularly Interspaced Short Palindromic Repeats associated system (CRISPR-Cas9). They offer techniques that manipulate genes with improved precision, targeting specific genes to more efficiently yield the desired characteristics in the new mutants.
CRISPR
One example utilises a group of bacterial endonuclease enzymes called CRISPR Associated Proteins. One is called Cas9 which has been demonstrated to identify specific gene sequences in plants and animals that then can be cut, modified, or switched on or off (gene silencing) by the Cas9 enzyme [6]. The CRISPR-cas9 gene editing technology was developed from a defence system that already exists in nature. Bacteria trigger an immune disease response when infected by viruses. They capture fragments of virus DNA and insert them into their own DNA in a specified sequence or pattern to produce segments known as CRISPR arrays. These templates act like a retained memory for when the bacteria are attacked again. The bacteria then use the modified DNA template (CRISPR arrays) to produce RNA segments that recognise sections of viral DNA to which they attach. This tag is used as a guide for the bacterial endonuclease enzymes, e.g. Cas9, to attack. The subsequent viral nucleotide sequence modification disables the virus. See Doudna and Sternberg (2018) for a clear, authoritative, and very readable account of the development of gene editing (GE) technologies, most notably CRISPR for which Jennifer Doudna shared the 2020 Nobel Prize for Chemistry. (See later section headed ‘Further Reading’)
With commercialised technology, there is no need for a transgene construct nor typically a need for inserting a selection marker. The Cas9 approach uses metabolic means to edit genetic codes. It provides a metabolic toolbox that can target and modify specific existing genetic sequences. It delivers the means to engineer the existing metabolism of crops and livestock with at least the same precision as transgenic methods. Genetic technologies have moved from a randomised classical approach to one of being targeted through the transfer of specific genes. Now, they can carry out the specific editing of existing DNA sequences through GE.
Current research includes controlling cyanide levels in processed cassava tubers to reduce the risk of accidental cyanide poisoning when consumed. Reducing the cyanogenesis in cassava is an active target of CRISPR gene editing [7]. A small number of GE crops are entering the market including a low oleic, low linolenic acid soybean oil cultivar Calyno (2019 in the USA) and a GE tomato (2020 in Japan) that is high in the amino acid gamma-aminobutyric acid (GABA) [8]. Five other GE crops were recently commercialized including, OSR, rice, maize, mushroom and camelina [9].
Emerging Gene Editing Tools
Base editing is a new Cas9 based development which can efficiently generate precise point mutations with minimal undesirable insertions or deletions [10,11]. ZFNs are a new GE tool used to effect small changes (gene correction) or transfer foreign DNA fragments (gene addition), often without extensive selection, into the chromosome. The technology has been applied to various targets from fruit flies, tobacco plants, maize, and human stem cells. Opportunities particularly lie in novel therapeutic solutions in human and animal health [12]. The use of Transcription Activator-like Effector Nucleases (TALENs) is a further enhancement of these site-specific GE approaches. These and other emerging tools will likely feature in future developments in food and feed supply where GE is adopted.
Precision Breeding Technologies and Molecular Breeding
Marker Assisted Selection (MAS) and Marker-Assisted Backcross Breeding (MABB) approaches do not require the use of GE or GM and enable the targeting of multiple genetic factors in parallel, for example, to optimise the nutritional properties of crops. Using MAS, a biofortified cultivar of maize has been developed with enhanced levels of vitamin-E, vitamin-A, lysine, and tryptophan to tackle malnutrition. This was achieved whilst maintaining the yield potential of the commercial parental cultivar. This was a more efficient operation by being able to identify markers within or closely linked to the genes targeted for selection using state-of-the-art analytical tools, genotyping and PCR. The mutants with the target genes responsible for elevated levels of nutrients were readily available in existing collections of cultivars. Subsequent marker assisted backcross breeding ensured the reconstructed hybrids retained the desired level of distinctness, uniformity, and stability [13]. In Australia, a Poll Gene Marker Test, has been developed. Hence, producers have a tool to identify breeding animals that consistently produce polled progeny (without horns), that will breed true, and carriers of horned genes. This avoids risks posed by handling horned cattle and makes redundant the practice of de-horning calves [14].
Somatic (asexual) hybridisation techniques were developed in the late 1970s / early 1980s. They often involve fusing the isolated cell protoplasts of individuals of two otherwise sexually incompatible species that possess traits of interest. Plants are then propagated from the subsequent tissue cultures that are generated. Resulting tissues or propagated plants can then be screened for preferred properties or marker genes that can be entered into conventional or MABB programmes. The recovery of fungal pathogen resistance from ancestral lines of modern potato varieties was achieved by hybridising Solanum bulbocastanum with a modern cultivar of S. tuberosum However, the technique has limited applications as there are a range of technical restrictions to achieving successful crosses [15].
Somatic mutations are genetic mutations that arise in the cells of organisms naturally. They are typically rare events that can be accelerated by exposure to radiation as described previously. However, when hybridised cells are subsequently propagated via tissue culture there is an increased rate of mutation resulting in somaclonal variation. This may result from a range of environmental factors impacting the differentiating clonal cells. [16] The new molecular tools can be used to rapidly screen cell cultures for emerging desirable mutations, or to screen out undesirable mutations. They can then be selected and either propagated directly, in the case of plant tissue culture, or used in GM or GE programmes of breed or strain improvement.
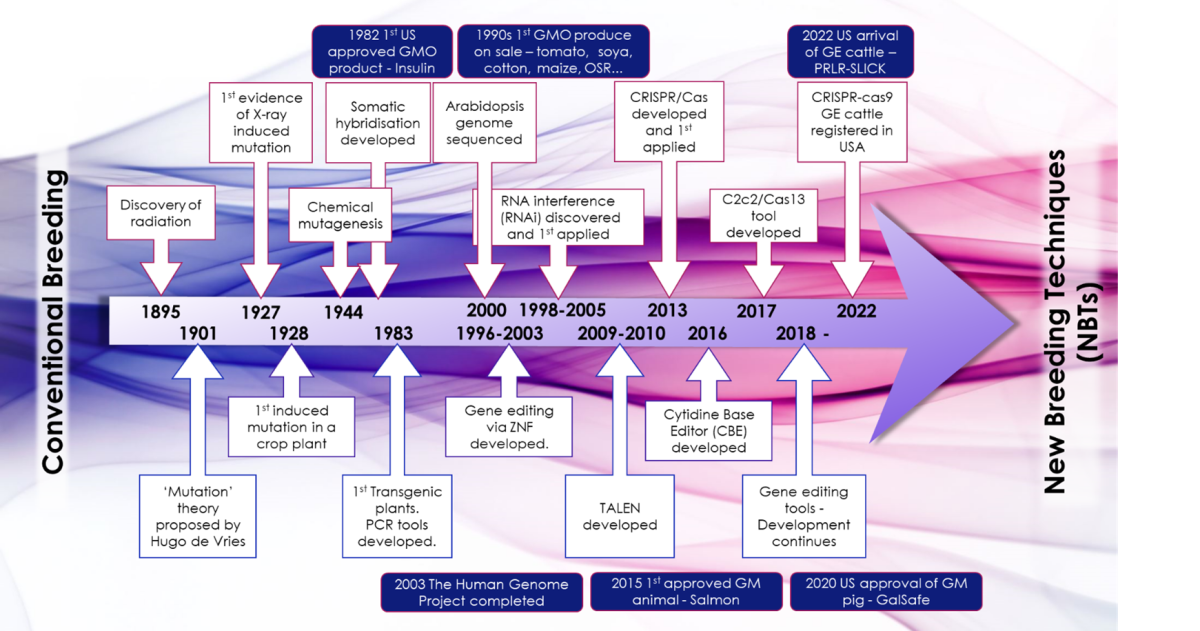
Figure 1 – Evolution of Genetic Modification Development for Agri-Food
These emerging molecular breeding tools will continue to develop and feed into food systems. Precision breeding and marker assisted selection will be particularly important where consumers oppose direct genetic interventions using GM or GE technologies.
The microbiome associated with the root systems of crop plants (mycorrhiza) can have a large impact in crop performance, yield, disease susceptibility and nutrient flux. Also, the ability for legumes to form nodules incorporating nitrogen fixing bacteria is a genetic trait restricted to relatively few crop species and is controlled by multiple genes. The high degree of genetic complexity of plant microbe interactions can now be unravelled at the genetic and biochemical level with the availability of new cost-effective molecular tools. There are opportunities for maximising crop yield and crop health by applying these NBTs to plant-rhizobia and nitrogen fixation systems. [17]
Consumers in the USA have access to corn-starch, corn syrup, corn oil, soybean oil, rapeseed oil, granulated sugar and a small range of fresh fruit and vegetables including potatoes, squash, apples, papayas, and pink pineapples from GM sources. However, the highest volumes of GMO crops grown in the United States are used for animal feed. [18]
New developments are not restricted to crops and livestock. We are in the middle of a boom in biotechnology that includes fermentation as a means of manufacturing food and feed ingredients or protein rich biomass at scale. The microorganisms used are typically yeast and bacteria already familiar to the food technologist and found in bread, beer, wine, cheese, yoghurt and other fermented foods. However, there is a rich diversity of fungi, algae and lesser-known yeast and bacteria to be explored for novel properties that meet current needs. The flavour and fragrance industry has an extensive portfolio of ingredients that are more frequently being manufactured using fermentation. [19]
The successful scaling up of biomanufacturing capacity reduces the risk of endangering native plant species that are not easily cultivated. This sector's expansion has been partly driven by the move to “natural” ingredients and “clean label” products and the demands for substituting the synthetic, often less expensive, counterparts. Although the definition of natural may differ from region to region globally the principle of using cells as factories is a common theme for most large producers. Microbial fermentation processes are now commonly used to facilitate previously difficult chemical transformations either by utilizing naturally present enzymes, either excreted from the cells or act in situ, to carry out chemical reactions from a feedstock to produce the ingredient of interest. This is then separated from the fermentation medium if excreted from the cells or extracted from the cells during downstream processing. There are many opportunities for gene manipulation in generating new properties in already food safe microorganisms to for example upregulate key metabolic pathways to improve process efficiency and yield or silence genes to halt the production of toxic by-products or anti-nutritional factors such as bitter or astringent metabolites. [20]
As early as 1992 the US FDA developed a policy that stated that foods from GMO sources must meet the same requirements, including the same safety standards, as foods derived from traditionally bred hybrids. Labelling of foods containing products from bioengineered crops or livestock was not required. However, in 2018 the US FDA announced the National Bioengineered Food Disclosure Standard, a national mandatory standard for disclosing foods that are or may be bioengineered. It defines bioengineered foods ‘as those that contain detectable genetic material that has been modified through certain lab techniques and cannot be created through conventional breeding or found in nature’. The standard came into force on 1 January 2022.
USDA Bioengineered (BE) Symbols:
images source link
Around 190 million hectares of GM crops are grown worldwide by over 17 million farmers in 29 countries. The vast majority of soy, maize and cotton in the USA is genetically modified.
The rate of the global rollout of NBTs and the benefits they can provide is limited largely by the restrictions imposed by regulations applied regionally. In some cases, regulations are determined through product-based scrutiny of the evidence supporting product safety and the assessment of risk to consumers and the environment. Across the EU, the UK and a selection of other countries, restrictions are based largely on the technology being used to develop new varieties and breeds by applying a precautionary principle in the absence of evidence of harm. In countries that do not permit GMOs to be produced, or for GM products or GM-derived ingredients to enter the supply chain, arguments persist as to whether NBTs, in particular gene editing, should fall under the umbrella term of GM. This is particularly problematic if NBT products cannot be distinguished from products derived from traditional mutagenesis and conventional breeding strategies. [21] Supply chain traceability will be critical if mandatory labelling is rolled out across the global marketplace.
The regulatory status of these emerging technologies in the UK is likely to be in flux for some time and will be reviewed and reported by the IFST as changes impacting food production, supply, labelling, and trade are rolled out. Some types of genome-edited plants are treated more like conventional varieties in some countries such as Australia, and Japan. However, of the 27 EU countries, 16 prohibit the use of GMOs, and products from genome editing may still be licenced as GMOs if there are no changes in EU policies to manage the regulation of GE specifically. The outcomes of a 2021 public consultation (England) on the regulation of genetic technologies, with a focus on GE, [22] concluded that the government “...will seek to bring forward primary legislation at a suitable opportunity to amend the regulatory definitions of a GMO to exclude organisms that have genetic changes that could have been achieved through traditional breeding or which could occur naturally. We will also consider the appropriate regulatory measures needed to enable gene edited crops equivalent to those produced through traditional breeding to be brought to market.” [23]
- Esquinas-Alcázar, J. (2005). Protecting crop genetic diversity for food security: political, ethical and technical challenges. Nat Rev Genet 6, 946–953 (2005). https://doi.org/10.1038/nrg1729.
- Kovalchuk, I (2016). Genome Stability: An Evolutionary Perspective. In Genome Stability: From Virus to Human Application. Igor Kovalchuk and Olga Kovalchuk, (Eds.) 2016, Pages 1-18 https://doi.org/10.1016/B978-0-12-803309-8.00001-X
- Khoury, C.K., Brush, S., Costich, D.E., Curry, H.A., de Haan, S., Engels, J.M.M., Guarino, L., Hoban, S., Mercer, K.L., Miller, A.J., Nabhan, G.P., Perales, H.R., Richards, C., Riggins, C. and Thormann, I. (2022), Crop genetic erosion: understanding and responding to loss of crop diversity. New Phytol, 233: 84-118. https://doi.org/10.1111/nph.17733
- Rocha, A.d.J.; Soares, J.M.d.S.; Nascimento, F.d.S.; Santos, A.S.; Amorim, V.B.d.O.; Ferreira, C.F.; Haddad, F.; Santos-Serejo, J.A.d.; Amorim, E.P. (2021). Improvements in the Resistance of the Banana Species to Fusarium Wilt: A Systematic Review of Methods and Perspectives. J. Fungi 2021, 7, 249. https://doi.org/10.3390/jof7040249
- Karki H. S., Shelly H., Jansky S.H., Halterman D.A. (2021). Screening of Wild Potatoes Identifies New Sources of Late Blight Resistance. Plant Disease 2021 105:2, 368-376 https://doi.org/10.1094/PDIS-06-20-1367-RE
- A Nobel Prize for genetic scissors. Nat. Mater. 20, 1 (2021). https://doi.org/10.1038/s41563-020-00895-z.
- Juma, B.S., Mukami, A., Mweu, C., Ngugi, M.P., Mbinda, W. (2022) Targeted mutagenesis of the CYP79D1 gene via CRISPR/Cas9-mediated genome editing results in lower levels of cyanide in cassava. Front. Plant Sci. 13:1009860. https://doi.org/10.3389/fpls.2022.1009860
- Takayama, M., Matsukura, C., Ariizumi, T., Ezura H. (2017). Activating glutamate decarboxylase activity by removing the autoinhibitory domain leads to hyper γ-aminobutyric acid (GABA) accumulation in tomato fruit. Plant Cell Rep 36, 103–116 (2017). https://doi.org/10.1007/s00299-016-2061-4
- Pixley, K.V., Falck-Zepeda, J.B., Paarlberg, R.L., Phillips, P.W.B., Slamet-Loedin, I.H., Dhugga, K.S., Campos, H., Gutterson, N. (2022). Genome-edited crops for improved food security of smallholder farmers. Nat Genet 54, 364–367 (2022). https://doi.org/10.1038/s41588-022-01046-7
- Wang F, Zeng Y, Wang Y, Niu Y. (2020). The Development and Application of a Base Editor in Biomedicine. Biomed Res Int. 2020 Aug 14; 2020:2907623. doi: 10.1155/2020/2907623
- Azameti M. K., Dauda W. P. (2021) Base Editing in Plants: Applications, Challenges, and Future Prospects. Frontiers in Plant Science, 12, 2021. https://doi.org/10.3389/fpls.2021.664997
- Urnov, F., Rebar, E., Holmes, M.H. Zhang S., Gregory P.D. (2010). Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11, 636–646 (2010). https://doi.org/10.1038/nrg2842
- Das, A.K, Gowda, M.M., Muthusamy, V., Zunjare R.U., Chauhan, H.S., Baveja, A., Bhatt, V., Chand, G., Bhat, J.S., Guleria S.K., Saha, S., Gupta, H.S., Hossain, F. (2021). Development of Maize Hybrids with Enhanced Vitamin-E, Vitamin-A, Lysine, and Tryptophan Through Molecular Breeding. Front Plant Sci. 2021 Jul 21;12:659381. doi:10.3389/fpls.2021.659381
- Lyons, R. and Randhawa, I. (2020) Improving the Australian Poll Gene Marker Test. Report published by Meat and Livestock Australia Limited. Link
- Sedlák, P., Sedláková, V., Vašek, J., Zeka, D., Čílová, D., Melounová, M., Orsák, M., Domkářová, J., Doležal, P., & Vejl, P. (2022). Phenotypic, molecular and biochemical evaluation of somatic hybrids between Solanum tuberosum and S. bulbocastanum. Sci Rep 12, 4484 (2022). https://doi.org/10.1038/s41598-022-08424-5
- National Research Council (US) Board on Agriculture. Genetic Engineering of Plants: Agricultural Research Opportunities and Policy Concerns. Washington (DC): National Academies Press (US); 1984. https://doi.org/10.17226/10.
- Shelake, R.M., Pramanik, D. & Kim, J. Y. (2019). Exploration of Plant-Microbe Interactions for Sustainable Agriculture in CRISPR Era. Microorganisms 2019, 7, 269. https://doi.org/10.3390/microorganisms7080269
- FDA website "GMO crops, animal food and beyond” https://www.fda.gov/food/agricultural-biotechnology/gmo-crops-animal-food-and-beyond/
- Verma, D.K., Al-Sahlany, S.T.G., Niamah, A.K., Thakur, M., Shah, N., Singh, S., Baranwal, D., Patel, A. R., Utama, G.L., Aguilar, C.N. (2022) Recent trends in microbial flavour Compounds: A review on Chemistry, synthesis mechanism and their application in food. Saudi Journal of Biological Sciences. Volume 29, Issue 3, March 2022, Pages 1565-1576 https://doi.org/10.1016/j.sjbs.2021.11.010
- Mannaa, M., Han, G., Seo Y-S., Park, I., Giavasis I. (2021) Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods. 2021 Nov; 10(11): 2861. https://doi.org/10.3390%2Ffoods10112861
- European Network of GMO Laboratories (ENGL), Detection of food and feed plant products obtained by new mutagenesis techniques, 26 March 2019 (JRC116289) https://gmo-crl.jrc.ec.europa.eu/doc/JRC116289-GE-report-ENGL.pdf
- DEFRA “The regulation of genetic technologies” A public consultation on the regulation of genetic technologies. January 2021. https://consult.defra.gov.uk/agri-food-chain-directorate/the-regulation-of-genetic-technologies/
- Genetic technologies regulation: government response DEFRA March 2021. https://www.gov.uk/government/consultations/genetic-technologies-regulation/outcome/genetic-technologies-regulation-government-response
- ARM Antimicrobial Resistance Marker
- ARMG Antimicrobial Resistance Marker Gene
- BE Bioengineered (USA)
- CBE Cytidine Base Editor (or BE Base Editor)
- CRISPR-Cas9 Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated system
- GE Gene Editing
- GM Genetically Modified
- GMO Genetically Modified Organism
- NBT New or Novel Breeding Techniques or Technologies
- NPBT New or Novel Plant Breeding Techniques
- PBT Precision Breeding Techniques
- PCR Polymerase Chain Reaction
- PIP Plant-Incorporated Protectant
- TALEN Transcription Activator-Like Effector Nuclease
- ZFN Zinc Finger Nuclease
- Glossary
- Agrobacterium tumefaciens mediated transformation (ATMT): a desired DNA fragment is incorporated into the bacteria’s cyclic DNA (plasmid), which is then used as a carrier to infect plants where the transformed plasmid transfers the DNA fragment into the recipient cell nucleus.
- Bioengineered (BE): US term commonly used for GMOs.
- Cas9: protein found in bacteria that helps defend against viruses. It can be easily adopted to target and bind to a DNA sequence of interest using an RNA fragment as a template. The DNA can then be cut, modified, or switched on or off (gene silencing).
- Cytidine Base Editor (CBE): a genome editing tool. CBE can induce direct conversion of base pairs cytosine (C) to thymine (T) or guanine (G) to adenine (A) at a programmable target locus.
- Epigenetics: the study of changes in the characteristics or traits of a cell or organism arising from changes in gene expression rather than changes in the DNA sequence itself. Especially where the changes are heritable. E.g. Post transcriptional or post translational modification.
- Gene drive:
i) process that promotes or favours the biased inheritance of certain genes from generation
to generation.
ii) any genetic element (naturally arising or engineered) able to bias its inheritance within a population
iii) tool to effect certain changes in a population. L.S. Alphey, A. Crisanti, F. Randazzo, and O.S. Akbari (2020). Standardizing the definition of gene drive. PNAS, December 8, vol. 117, no. 49 30864–30867 http://www.pnas.org/cgi/doi/10.1073/pnas.2020417117)
- Genetic Engineering: sometimes abbreviated as GE e.g. in USA
- Genetically Modified Organism (GMO): organisms in which the genetic material (DNA) has been altered in a way that does not occur naturally by mating or natural recombination.
- Biolistic or ‘gene gun’: method that uses a small particle of gold or tungsten coated with DNA fragments fired into cells. The nucleus can take up the DNA and genetic transformation occurs when the foreign DNA is integrated as part of the organisms own genomic sequence
- Gene or Genome Editing (GE): represents a group of technologies that can change an organism's DNA or how it is expressed. These technologies allow genetic material to be added, removed, or modified at specified locations in the genome
- Post transcriptional modification: in situ changes to RNA including methylation and glycation
- Post translation modification: in situ changes to proteins following biosynthesis from mRNA that affects expression, for example: glycosylation, acetylation and alkylation including methylation.
- Zinc Finger Nuclease (ZFN): gene editing tool involving targeted genome cleavage by engineered sequence-specific ZFNs, followed by gene modification during subsequent repair. Small changes (gene correction) or foreign DNA fragments (gene addition) can be transferred, often without selection, into the chromosome.
The Bio Cassava Plus Program: Biofortification of Cassava for Sub-Saharan Africa (2011). Sayre, R., Beeching, J.R., Cahoon, E.B., Egesi, C., Fauquet, C., Fellman, J., Fregene, M., Gruissem, W., Mallowa, S., Manary, M., Maziya-Dixon, B., Mbanaso, A., Schachtman, D.P., Siritunga, D., Taylor, N., Vanderschuren, H., Zhang, P. Annual Review of Plant Biology. Vol. 62:251-272. https://doi.org/10.1146/annurev-arplant-042110-103751
‘The Golden Rice Tale’ by Ingo Potrykus. Published online by AgBioWorld (2011). http://www.agbioworld.org/biotech-info/topics/goldenrice/tale.html
Genetically Engineered Crops: Experiences and Prospects. National Academies of Sciences, Engineering, and Medicine. (2016). Washington, DC: The National Academies Press. doi.org/10.17226/23395.
A Crack in Creation. The New Power to Control Evolution. Jennifer Doudna and Samuel Sternberg. Published by Vintage. 14th June 2018. 304 pages. ISBN10 1784702765
New breeding technique "genome editing" for crop improvement: applications, potentials and challenges. Aglawe S.B., Barbadikar K.M., Mangrauthia S.K., Madhav M.S. (2018) 3 Biotech. 2018 Aug;8(8):336. doi.org/10.1007%2Fs13205-018-1355-3
Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier ISAAA Brief 55-2019: Executive Summary
https://www.isaaa.org/resources/publications/briefs/55/executivesummary/default.asp
Evolution of plant mutagenesis tools: a shifting paradigm from random to targeted genome editing. Shelake, R.M., Pramanik, D. & Kim, JY. Plant Biotechnol Rep 13, 423–445 (2019). doi.org/10.1007/s11816-019-00562-z
Biotechnology and Genetically Modified Organisms in Food Labelling Requirements (2021) Luke Grocholl. Perfumer and Flavourist. Vol. 46, January 2021. Page 34-38. https://perfumerflavorist.texterity.com/perfumerflavorist/january_2021/MobilePagedReplica.action?pm=2&folio=34#pg37
Advances in Crop Breeding Through Precision Genome Editing. Nerkar G., Devarumath S., Purankar M., Kumar A., Valarmathi R., Devarumath R. and Appunu C. (2022) Front. Genet. 13:880195. https://doi.org/10.3389/fgene.2022.880195
Base Edit Your Way to Better Crops. Eisenstein M. (2022). Technology Feature, Nature 604, 790-792 (2022) doi.org/10.1038/d41586-022-01117-z
E-seminar: Screening for GMOs in consignments of rice and rice products. Produced by LGC Academy (2022).
Genome-edited crops and 21st-century food system challenges. EPRS | European Parliamentary Research Service Scientific Foresight Unit (STOA) PE 690.194 – July 2022 https://doi.org/10.2861/290440
Genome-Edited Food Crops. POST NOTE Number 663 January 2022 The Parliamentary Office of Science and Technology, Westminster, London SW1A 0AA https://researchbriefings.files.parliament.uk/documents/POST-PN-0663/POST-PN-0663.pdf
Harnessing bioengineered microbes as a versatile platform for space nutrition. Llorente, B., Williams, T.C., Goold, H.D., Pretorius I.S., Paulsen I.T. (2022). Nat Commun 13, 6177 (2022). https://doi.org/10.1038/s41467-022-33974-7
Institute of Food Science & Technology has authorised the publication of the following Information Statement on Beyond Genetic Modification: GM, Gene Editing and New Breeding Technologies.
This Information Statement has been prepared by Dr Craig Duckham FIFST and Dr Wayne Martindale FIFST and peer-reviewed and approved by the IFST Scientific Committee. This Information Statement is dated February 2023.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
