
July 2024
Executive Summary
- The term dioxins includes polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF) and dioxin-like polychlorinated biphenyls (DL-PCB).
- Dioxins are ubiquitous, persistent and highly toxic environmental contaminants, which can compromise the reproductive and developmental systems, and can also interfere with hormone activity.
- PCDD and PCDF are not commercially manufactured. They are by-products in the manufacture of organochlorine chemicals and are also produced during the combustion of chlorine-containing waste.
- PCB (including DL-PCB) were widely used in the manufacture of electrical, heat transfer and hydraulic equipment, until their production was abandoned in most countries in the 1970s.
- The most significant exposure route for most people is via their diet.
- Dioxins are found at low levels in all foods, especially oily or fatty food of animal origin from species close to the top of the food chain.
- Levels of dioxins are decreasing as pollution control measures, introduced some decades ago, have an impact on the environment.
Keywords: dioxins, PCB, POP, risk assessment, dietary exposure, incidents, food contaminants, environmental contaminants
Dioxins and dioxin-like compounds (DLC) are members of a group of chemical compounds that are described as persistent organic pollutants (POP). Some POP are highly toxic and carcinogenic, although their toxicity varies greatly.
The POP addressed in this information statement are:
- Polychlorinated dibenzo-p-dioxins (PCDD)
- Polychlorinated dibenzofurans (PCDF)
- Dioxin-like polychlorinated biphenyls (DL-PCB)
PCDD, PCDF and DL-PCB are three of the so-called ‘dirty dozen’ compounds identified as candidates for control and elimination after the institution of the Stockholm Convention on POP in 2001. They are collectively referred to as ‘dioxins’ within this information statement.
PCDD and PCDF are unintentional by-products of human activity, especially the combustion of chlorine-containing compounds.
PCB (including DL-PCB) are non-flammable and chemically stable with a high boiling point and electrical insulating properties. Consequently, although their production was abandoned in most countries in the 1970s, PCB were widely used in electrical, heat transfer and hydraulic equipment, and a variety of other applications.
Dioxins occur in the environment throughout the world and accumulate in the food chain, mainly in the fatty tissue of animals. Animal feeds and feed additives are major sources of contamination for food of animal origin, including eggs, meat, fish and milk. They are highly toxic and can compromise the reproductive, developmental and endocrine systems. Given their toxicity, levels of dioxins in feed and food are regulated in Europe, the USA and other countries. Consequently, reliable methods are required for the sampling and analysis of dioxins.
PCDD and PCDF are a group of relatively stable lipophilic organic substances comprising two groups of tricyclic planar compounds (Figure 1). Depending on the number of chlorine atoms and their positions in the rings, 75 PCDD and 135 PCDF (termed ‘congeners’) can occur. Only 17 of these are relatively persistent in animals and humans and therefore considered relevant.
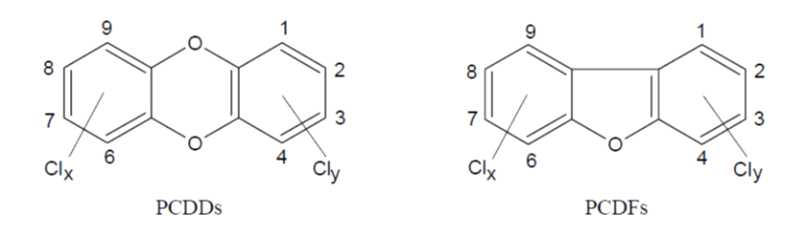
Figure 1. Structure of PCDD and PCDF: Clx + Cly = 1-8.
PCDD and PCDF are unintentional by-products of human activity which have never been produced as intentional end products, and have no technological use. For example, they are produced by waste incineration, by burning biomass fuels, during iron ore sintering, and by forest fires. The production of dioxins is facilitated by the presence of chlorine and/or copper (as a catalyst), in the combustible material. The formation of dioxins can be enhanced by the presence of contaminants in biomass, including pesticides, wood preservatives and paint, which can introduce additional chlorine, metal catalysts, and even dioxin-precursors into combustion systems. PCCD and PCDF are also present in the effluent discharge of pulp and paper mills, as a result of chlorine bleaching, and are also produced in the manufacture of some organochlorine chemicals when these processes are not effectively controlled.
DL-PCB are a sub-group of the wider class of PCB which demonstrate a similar toxicity to PCDD and PCDF.
PCB are a group of organochlorine compounds that are non-flammable and chemically stable with a high boiling point and good electrical insulating properties. They are synthesised by the catalysed chlorination of biphenyl. Depending on the number of chlorine atoms (1–10), and their position in the two rings, 209 different congeners are possible. Figure 2 shows the structure of PCB and the numbering of the carbon atoms in the two rings.
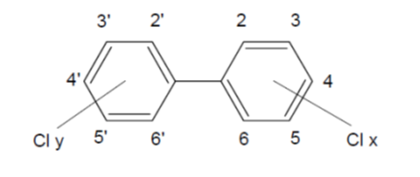
Figure 2. Structure of PCB: Cly + Clx = 1-10.
In contrast to PCDD and PCDF, PCB had widespread use in numerous industrial applications, generally in the form of complex technical mixtures. They were produced with an estimated total world production of 1.2–1.5 million tonnes between 1929 and the end of the 1970s, when their production was abandoned in most countries, due to concerns about their toxicity and persistence in the environment and biota. Because of their physicochemical properties, PCB were widely used in a number of industrial and commercial applications. Technical PCB mixtures were mobile oils, viscous liquids or sticky resins, depending on the degree of chlorination (21–68% chlorine). Various commercial mixtures were produced with different trade names, such as Aroclor, Kanechlor, Declor and Clophen. PCB were used as insulating fluid in electrical transformers, and as heat exchange fluids. As a result of their widespread use, leakages, improper disposal practices and persistence, PCB also have a global distribution in the environment. Depending on the number and position of the chlorine atoms in the molecule, some of them are poorly degraded and, due to their lipophilic properties, are bioaccumulated in the food chain.
The PCB Elimination Network (PEN) develops and implements an awareness raising strategy, including videos, website, webinars and fact sheets, to ensure that PCB remain on the international agenda. It also supports PCB activities within the Basel, Rotterdam and Stockholm Conventions Conferences, and the United Nations Environment Assembly.
Dioxins deposited on land and in waterways, in the form of contaminated soil (e.g. pastureland) and/or contaminated animal feed, are ingested by a variety of animals used as human food. Given their lipophilic nature, dioxins accumulate in the fat tissues of animals and some organs. (Fries, 1995). Proteins in the liver, for example, can bind dioxins.
Animal feed from a variety of origins may be contaminated, including plants, animals, minerals, fish and feed additives. Food of animal origin which may be contaminated by dioxins includes meat, animal products (eggs, cows’ milk, from sheep, pigs, poultry), fish and eels. Dioxins may also occur in cereals, oilseeds, fruit and vegetables, including fresh herbs.
Dioxins in the food chain are of concern because of a number of adverse health effects that can occur at very low levels of exposure. Acute exposure at relatively high level can result in chloracne (a skin condition). Of perhaps greater concern are the chronic effects of low-level exposure, typically through the diet, which can cause reproductive, carcinogenic and other effects (Faqi et al, 1998; COC, 2001).
Toxic Equivalency Factors (TEF) and Toxic Equivalents (TEQ)
The most toxic dioxins are 2,3,7,8-TCDD and 1,2,3,7,8-PeCDD, which are given Toxic Equivalency Factors (TEF) of 1. All other dioxins are compared to these and given comparative TEF (see Table 1).
In order to compare the toxicity of a mixture of congeners, Toxic Equivalents (TEQ) based on the TEF of the individual congeners are calculated. This scheme assumes that the relevant PCDD, PCDF and DL-PCB bind to the intracellular aryl hydrocarbon receptor (AHR) and cause the same type of AHR-mediated biochemical and adverse effects. Another important requirement of the TEQ concept is the persistence and accumulation of the compounds in the body.
To calculate the total TEQ value of a sample, the concentration of each congener is multiplied by its TEF and the products are then added together. The resulting TEQ value expresses the toxicity of PCDD, PCDF and DL-PCB in a complex sample relative to the two most toxic dioxins.
The TEQ value is calculated as follows: TEQ = ∑ [PCDDi · TEFi] + ∑ [PCDFi · TEFi] + ∑ [PCBi · TEFi].
The current TEF values were proposed by the World Health Organisation (WHO) in 2005 (van den Berg et al, 2006) and are termed WHO2005-TEF based on the year of the WHO expert meeting (Table 1).
Table 1. WHO Toxic equivalency factors (TEF) established in 2005 and compared with WHO TEF proposed in 2022 (DeVito et al, 2024).
|
Congener |
2005 WHO-TEF |
2022 WHO-TEF |
|
Dioxins |
|
|
|
1,2,3,7,8-PeCDD |
1 |
0.4 |
|
1,2,3,4,7,8-HxCDD |
0.1 |
0.09 |
|
1,2,3,6,7,8-HxCDD |
0.1 |
0.07 |
|
1,2,3,7,8,9-HxCDD |
0.1 |
0.05 |
|
1,2,3,4,6,7,8-HpCDD |
0.01 |
0.05 |
|
OCDD |
0.0003 |
0.001 |
|
Furans |
|
|
|
TCDF |
0.1 |
0.07 |
|
1,2,3,7,8-PeCDF |
0.03 |
0.01 |
|
2,3,4,7,8-PeCDF |
0.3 |
0.1 |
|
1,2,3,4,7,8-HxCDF |
0.1 |
0.3 |
|
1,2,3,6,7,8-HxCDF |
0.1 |
0.09 |
|
1,2,3,7,8,9-HxCDF |
0.1 |
0.2 |
|
2,3,4,6,7,8-HxCDF |
0.1 |
0.1 |
|
1,2,3,4,6,7,8-HpCDF |
0.01 |
0.02 |
|
1,2,3,4,7,8,9-HpCDF |
0.01 |
0.1 |
|
OCDF |
0.0003 |
0.002 |
|
PCBs |
|
|
|
PCB77 |
0.0001 |
0.0003 |
|
PCB81 |
0.0003 |
0.006 |
|
PCB126 |
0.1 |
0.05 |
|
PCB169 |
0.03 |
0.005 |
|
MONO-ORTHO PCBs |
|
|
|
PCB105 |
0.00003 |
0.00003 |
|
PCB114 |
0.00003 |
0.00003 |
|
PCB118 |
0.00003 |
0.00003 |
|
PCB123 |
0.00003 |
0.00003 |
|
PCB156 |
0.00003 |
0.00003 |
|
PCB157 |
0.00003 |
0.00003 |
|
PCB167 |
0.00003 |
0.00003 |
|
PCB189 |
0.00003 |
0.00003 |
The latest TEF values (WHO2022-TEF) were proposed by the World Health Organization (WHO) in 2022 following rigorous expert and statistical evaluation of an expanded set of studies of the congeners’ toxicological effects (DeVito et al, 2024). The WHO2022-TEF are listed in Table 1. They are expected to supersede the TEF derived in 2005.
Older analytical data generated before 2005 is generally reported as WHO1998-TEQ, I-TEQ or Nordic-TEQ. When interpreting TEQ results and evaluating, for example trends in the levels or exposure, it is important to know which TEF were used.
The risk for animal and human health related to the presence of dioxins and DL-PCB in feed and food was evaluated by the EFSA Panel on Contaminants in the Food Chain in 2018 (EFSA, 2018). These were the terms of reference:
• evaluation of the toxicity of dioxins and DL-PCB for animals and humans, considering all relevant adverse acute and chronic health effects
• estimation of the dietary exposure (chronic and, if relevant, acute dietary exposure) of the EU population including the consumption patterns of specific (vulnerable) groups of the population (e.g. high consumers, children, people following a specific diet, etc.)
• estimation of the exposure of the different animal species to dioxins and DL-PCBs from feed and the levels of transfer/carry-over from the feed to the products of animal origin for human consumption
• assessment of the chronic (and if relevant acute) human health risks for the EU population including for specific (vulnerable) groups of the population as the consequence of the estimated dietary exposure
• assessment of the animal health risks for the different animal species as the consequence of the estimated exposure from animal feed.
Selected outputs from the EFSA Panel’s evaluation are reported in the sections below.
Having reviewed the data from experimental animal and epidemiological studies, EFSA decided to base the human risk assessment on effects observed in humans, and to use animal data as supportive evidence.
The critical effect was on semen quality following pre- and postnatal exposure.
The critical study showed a NOAEL of 7.0 pg WHO2005-TEQ/g fat in blood sampled at age 9 years, based on PCDD and PCDF TEQ. No association was observed when including DL-PCB TEQ.
Using toxicokinetic modelling, and taking into account the exposure from breastfeeding, and a twofold higher intake during childhood, it was estimated that daily exposure in adolescents and adults should be below 0.25 pg TEQ/kg bw/day. The Panel established a tolerable weekly intake (TWI) of 2 pg TEQ/kg bw/week. This was a seven-fold reduction on the previous TWI established by the Scientific Committee for Food in 2001.
The most comprehensive estimates of dietary exposure have been provided by EFSA (EFSA, 2012, 2018). In their update of the monitoring of dioxins and PCB levels in food and feed (EFSA, 2012), a total of 13,797 samples were assessed for dioxins and DL-PCB and 19,181 samples for non-dioxin-like PCB (NDL-PCB). These samples were submitted between 1995 and 2010 by 26 European countries. At least one quantified congener of dioxins and DL-PCB was found in almost all samples, whereas at least one NDL-PCB indicator was quantified in 68.4 % of the feed and 82.6 % of the food samples. Eels and fish liver, and derived products, contained the highest average contamination levels of both dioxins and PCB. Depending on the population group, defined as the combination of age class and the respective survey, average exposure to dioxins and DL-PCB was estimated to be between 0.57 and 2.54 pg WHO2005-TEQ/kg bw per day, and the 95th percentile between 1.2 and 9.9 pg WHO2005-TEQ/kg bw per day. Average exposure to NDL-PCB indicators was estimated to be between 4.3 and 25.7 ng/kg bw per day and the 95th percentile between 7.8 and 53.7 ng/kg bw per day. Fish, meat and dairy products appeared to be the highest contributing food groups to dietary exposure. Although the concentrations measured in foods are all extremely low in absolute terms, they are relatively high when compared with the various tolerable daily (or weekly or monthly) intake values that have been established by various organisations, including the WHO, in order to protect human health.
The TEF scheme does not currently cover other chemicals that have the same toxic mode of action, such as brominated and mixed halogenated analogues, and so may underestimate the true exposure to the totality of compounds that may contribute to this type of toxicity.
Global surveys of the occurrence of PCDD, PCDF and PCB in human milk have been performed by the WHO/UNEP (United Nations Environment Programme) since 1987. The three most recent surveys, from 2000 to 2010, have been reviewed (Van den Berg, 2016), and large global and regional differences were observed:
- levels of PCDD and PCDF were highest in India and some European and African countries. PCB levels were highest in East and West Europe,
- a temporal downward trend for PCDD, PCDF and PCB was indicated, and
- a risk-benefit assessment indicated that human milk levels of PCDD, PCDF and PCB were still significantly above those considered toxicologically safe. With respect to potential adverse health effects, a more dominant role of in utero exposure versus lactational exposure was indicated. If potential adverse effects were balanced against positive health aspects for (breastfed) infants, the advantages of breastfeeding far outweighed the possible disadvantages.
In the 2018 EFSA evaluation (EFSA, 2018), human chronic dietary exposure to PCDD, PCDF and DL-PCB was estimated using a data set containing:
- 19,965 food samples with all 29 congeners determined (17 PCDD/PCDFs and 12 DL-PCBs).
- 20,273 food samples with all 17 PCDD/PCDF congeners determined (including samples with the 29 congeners).
- 22,974 food samples with all 12 DL-PCB congeners determined (including samples with the 29 congeners).
An analysis of occurrence and consumption data from European countries indicated a mean total TEQ intake by Adolescents, Adults, Elderly and Very Elderly varied between 2.1 and 10.5 pg WHO2005-TEQ/kg bw/week. The P95 total TEQ intake for the same groups varied between 5.3 and 30.4 pg WHO2005-TEQ/kg bw/week. Both ranges imply a considerable exceedance of the TWI. Toddlers and Other Children showed a higher exposure than older age groups, and this was accounted for when deriving the TWI.
The main contributors to the mean dietary exposure, broken down by age group, were:
- Infants: Butter and Butter Oil (contributing 6.1-19.6%) and Fatty Fish (contributing 5.8-26.3%).
- Toddlers: Fatty Fish (contributing 5.9-13.9%), Cheese (contributing 5.9-21.8%) and Livestock Meat (contributing 7.7-16.2%).
- Other Children, Adolescents, Adults and Elderly: Fatty Fish (up to 56% contribution), Unspecified Fish Meat (up to 53.4% contribution), Cheese (up to 21.8% contribution) and Livestock Meat (up to 33.8% contribution).
Regulatory control
Regulatory limits for PCDD, PCDF and PCB in foods are in force in the UK under assimilated EU Regulation 1881/2006. Assimilated EU Regulation 2017/644 gives the official methods of sampling and analysis. The respective regulations in force in the EU are Commission Regulations (EU) 2023/915 and 2017/644. PCDD, PCDF and PCB in animal feed are regulated under the ‘Undesirable substances in products intended for animal feed’ Commission Directive 2002/32/EC.
Official control for dioxins and PCBs in food and feed in the EU is managed through a network of National Reference Laboratories (NRLs) and regional official control laboratories. The EU Reference Laboratory for halogenated POPs is the Chemisches und Veterinäruntersuchungsamt (State Institute for Chemical and Veterinary Analysis of Food), Freiburg, Germany. In the UK, Fera is the National Reference Laboratory for halogenated POPs in food and feed.
Preventing and reducing dioxins and dioxin-like compounds in food
The Codex Alimentarius Commission recommends the implementation of control measures at the feed level to reduce the contamination of food of animal origin (Codex Alimentarius, 2018). According to Codex:
“These may involve developing Good Agricultural Practice, Good Animal Feeding Practice and Good Manufacturing Practice guidance and measures to effectively reduce dioxins and PCBs in feed, including:
-
Identification of agricultural areas with increased dioxin and PCB contamination due to local emission, accidents or illegal disposal of contaminated materials, and monitoring of feed and feed ingredients derived from these areas,
-
Monitoring of dioxin and PCB content of sewage sludge and compost used as fertilizers in agriculture, as well as its compliance with nationally established guideline or maximum levels.
-
Establishing recommendations for special agricultural use (e.g. limitation of grazing or use of appropriate agricultural techniques),
-
Identification of possibly contaminated feed and feed ingredients,
-
Monitoring compliance with nationally established guideline levels or maximum levels, if available, and minimizing or decontaminating (e.g. refining of fish oil) non-complying feed and feed ingredients, and
-
Identification and control of critical feed manufacturing processes (e.g. artificial drying by direct heating).
Similar control measures, where applicable, should be considered for reducing dioxins and PCBs in food.”
To reduce exposure to dioxins and PCBs from wild-caught fish, lakes, rivers and marine catch areas that are heavily contaminated should be identified, along with relevant fish species. Fishing for relevant species in these areas should be controlled (Codex Alimentarius, 2018).
Dioxins and other contaminants can be controlled effectively by implementing Hazard Analysis and Critical Control Point (HACCP) programmes (Ahmad et al, 2019).
Sampling and analysis methods
As with all chemical hazards, the effective control of dioxins requires efficient surveillance and monitoring programmes which, in turn, require efficient sampling and analysis methods. Furthermore, surveillance programmes require the careful selection of representative populations and samples.
Analytical procedures for dioxins and PCBs in foods and feeds need to be highly accurate, highly specific and capable of achieving a low working range and low limits of quantification (Regulation (EU) 2017/644). Techniques using gas chromatography-mass spectrometry (GCMS), coupled with extensive clean-up/concentration, are used to achieve the required detection limits. Gas chromatography in conjunction with high-resolution mass spectrometry, or quadrupole high-resolution time-of-flight mass spectrometry, are examples of the GCMS procedures employed (Špánik and Machyňáková, 2017). Such measurements are expensive although prices have been decreasing in recent years. The high cost of analysis is the main reason why available data on dioxin levels in foods is limited.
Yeast-based in vitro screens combined with high-performance thin-layer chromatography have also been employed for the analysis of dioxin-like effects (Riegraf et al, 2019).
There are several examples whereby food has become contaminated by dioxins and PCBs and many of these are discussed by Hoogenboom et al. (2015):
- A nationwide survey of dioxins in foodstuffs in the USA, in the late 1990s, detected elevated levels of dioxins in some samples of poultry meats. Animal feed was implicated as the likely source of contamination, due to incorporation of ball clay (an anti-caking agent) in the production of the feeds. Control centred upon initially changing certain production practices to minimise the issue and subsequently in providing appropriate general advice, to the feed industry, regarding suitable monitoring to prevent repetition.
- A programme of dioxin monitoring in foods, in Germany in the 1990s, initially showed a gradual decline which was followed by a gradual increase in milk, butter and meats. Investigation implicated contaminated citrus pulp (a feed ingredient for ruminants) being supplied from South America. Once again, changes in production practices of the feed material eliminated the issue. This particular incident affected other European countries, as well as Germany, which highlighted the international aspect of the situation, as well as emphasising the need for regularly analysing large numbers of food samples for dioxins in order to detect shifts in trends including seasonal variations.
- An outbreak of illness in poultry, in Belgium in 1999, was traced to animal feed contaminated with dioxins in recycled fat used in its manufacture. Farms in France, Holland and Spain had also used the contaminated material for their livestock. Products from poultry and pigs were affected but not those from cattle. Farms were quarantined, and contaminated products destroyed. More than 30 countries temporarily banned certain food imports from Belgium, until the issue was resolved.
- Ireland experienced trouble with elevated dioxin levels in some pork products in 2008. Again, contaminated animal feed was implicated. The cause of the contamination was thought to be fuel oil used in the drying of the affected feed.
In all these cases, issues were initially raised by the finding of elevated dioxin levels in food products during routine random testing.
In 2019, the environmental group International Pollutants Elimination Network (www.ipen.org) reported elevated levels of dioxins and PCBs in free-range chicken eggs in Tropodo, Indonesia. The nearby burning of plastic as fuel was the likely source of the contamination.
PCDD, PCDF and PCB are ubiquitous contaminants and are found at low levels in all the food we eat. Concentrations are decreasing as a result of pollution control measures and increased food controls. Some other classes of chemical exhibit similar toxic action and these are less well-controlled and monitored, meaning there is a possibility we may be underestimating the total exposure to this type of toxic chemical.
The industry needs to be vigilant at all times and have sufficiently robust monitoring in place so that emerging issues can be resolved before products reach the marketplace.
PCDD polychlorinated dibenzo-p-dioxin
PCDF polychlorinated dibenzofuran
PCB polychlorinated biphenyl
DL-PCB dioxin-like PCB
NDL-PCB non-dioxin-like PCB
POP persistent organic pollutant
EU-RL European Union Reference Laboratory
NRL National Reference Laboratory
WHO World Health Organisation
TEQ toxic equivalence
TEF toxic equivalency factor
TWI tolerable weekly intake
AHR aryl hydrocarbon receptor
GCMS gas chromatography–mass spectrometry
Ahmad S, Masood F, Khatoon K, Malik A (2019). Risk management of chemical hazards arising during food manufacturing. In: Health and safety aspects of food processing technologies, 403–418. Springer International Publishing. http://dx.doi.org/10.1007/978-3-030-24903-8_13
Codex Alimentarius Commission (2018). Code of Practice for the prevention and reduction of dioxins, dioxin-like PCBs and non-dioxin-like PCBs in food and feed. CXC 62-2006.
DeVito M, Bokkers B, van Duursen MBM, van Ede K, Feeley M, Antunes E, Gáspár F, Haws L, Kennedy S, Peterson RE, Hoogenboom R, Nohara K, Petersen K, Rider C, Rose M, Safe S, Schrenk D, Wheeler MW, Wikoff DS, Zhao B, van den Berg M (2024). The 2022 World Health Organization reevaluation of human and mammalian toxic equivalency factors for polychlorinated dioxins, dibenzofurans and biphenyls. Regulatory and Toxicological Pharmacology 146. https://doi.org/10.1016/j.yrtph.2023.105525
EFSA Panel on Contaminants in the Food Chain, Knutsen HK, Alexander J, Barregard L, Bignami M, Bruschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl-Kraupp B, Hogstrand C, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A-C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Furst P, Hakansson H, Halldorsson T, Lundebye A-K, Pohjanvirta R, Rylander L, Smith A, van Loveren H, Waalkens-Berendsen I, Zeilmaker M, Binaglia M, Gomez Ruiz J A, Horvath Z, Christoph E, Ciccolallo L, Ramos Bordajandi L, Steinkellner H and Hoogenboom LR (2018). Scientific Opinion on the risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA Journal 16(11), 5333. [331 pp.] https://doi.org/10.2903/j.efsa.2018.5333
European Food Safety Authority (EFSA) (2012). Update of the monitoring of dioxins and PCBs levels in food and feed. EFSA Journal 10(7), 2832. [82 pp.] https://doi.org/10.2903/j.efsa.2012.2832
Faqi AS, Dalsenter PR, Merker HJ, Chahoud I (1998). Reproductive toxicity and tissue concentrations of low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male offspring rats exposed throughout pregnancy and lactation. Toxicology and Applied Pharmacology 150, 383–392. http://www.ncbi.nlm.nih.gov/pubmed/9653070
Fries GF (1995). A review of the significance of animal food products as potential pathways of human exposures to dioxins. Journal of Animal Science 73(6), 1629–1650. http://www.ncbi.nlm.nih.gov/pubmed/7673057
Hoogenboom R, Traag W, Fernandes A, Rose M (2015). European developments following incidents with dioxins and PCBs in the food and feed chain. Food Control 50, 670–683. https://doi.org/10.1016/j.foodcont.2014.10.010
Riegraf C, Reifferscheid G, Belkin S, Moscovici L, Shakibai D, Hollert H, Buchinger S (2019). Combination of yeast-based in vitro screens with high-performance thin-layer chromatography as a novel tool for the detection of hormonal and dioxin-like compounds. Analytica Chimica Acta 1081, 218–130. http://dx.doi.org/10.1016/J.ACA.2019.07.018
Špánik I, Machyňáková A (2017). Recent applications of gas chromatography with high-resolution mass spectrometry. Journal of Separation Science 41(1), 163–179. http://dx.doi.org/10.1002/jssc.201701016
Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE (2006). The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological Sciences 93(2), 223–241. https://doi.org/10.1093/toxsci/kfl055
Van den Berg M, Kypke K, Kotz A, Tritscher A, Lee SY, Magulova K, Fielder H, Malisch R (2017). WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit–risk evaluation of breastfeeding. Archives of Toxicology 91(1) 83–96. http://dx.doi.org/10.1007/s00204-016-1802-z
Institute of Food Science & Technology has authorised the publication of the following updated Information Statement on Dioxins and Dioxin-like Compounds in Foods and Feeds, peer-reviewed by professional members of IFST and approved by the IFST Scientific Committee.
IFST Scientific Committee is grateful to Professor Raymond Coker for valuable suggestions for this Information Statement.
This information statement is dated July 2024, replacing that of October 2020.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
