
October 2020
Cannabidiol (CBD) is a natural cannabinoid found within the cannabis plant and has been extracted and purified for sale as an active pharmaceutical ingredient (API) or a food supplement. CBD can be derived from most parts of Cannabis satvia plants. They are selectively extracted to concentrate the CBD and remove or reduce other chemical components. This process means the final product is different from traditional “hemp”. The whole, unrefined seeds, leaves or roots of Cannabis satvia plants, commonly known as hemp, have long been used in food, food supplements, rope, clothing, paper, board, soaps, and other cosmetics. CBD, on the other hand, is a specific compound extracted from Cannabis satvia which is now being used as an ingredient in a range of food products such as oils, confectionery, bakery products and drinks, in cosmetics such as creams, shampoos, bath products, toothpastes and deodorants, and in some medicinal products.
The World Health Organisation considers CBD to be generally well tolerated with a good safety profile for use in medicines. However, there may be adverse effects as a result of drug-drug interactions between CBD and patients’ existing medications. CBD containing products taken for the purpose of prevention or treatment of disease should be treated as medicines and need to be formally approved and licensed as such.
The regulatory status of CBD in non-medicinal products is unclear in many parts of the world. In the EU and UK, CBD was confirmed as a novel food product in January 2019. However, hemp itself and related products such as hemp seeds and cold-pressed hemp oils are not novel foods because there is evidence to show a history of consumption in the EU before May 1997. In the UK the Food Standards Agency (FSA) is confirmed that from 31 March 2021 only CBD containing food products which have submitted a valid novel foods application will be allowed to remain on the market.
In the USA, the Federal prohibition of hemp and CBD in food production was lifted by the 2018 Agriculture Improvement Act which excluded hemp from the definition of marijuana. Hemp is a valuable agricultural commodity and contains only trace levels of tetrahydrocannabinol (THC), the intoxicating compound in marijuana. Hemp with no more than 0.3% THC is no longer considered a controlled substance under the Controlled Substance Act in the USA. However, in contrast Food Drug Administration (FDA) explicitly retains jurisdiction to regulate the use of CBD in food, beverages, dietary supplements and other FDA-regulated products. Therefore, CBD remains restricted or prohibited in food within some US States. It is largely unrestricted in others.
The UK FSA have advised that those who are pregnant, breastfeeding or taking any medication should not consume CBD products. Healthy adults are also advised to think carefully before taking CBD, and the FSA recommends no more than 70mg a day (about 28 drops of 5% CBD) unless under medical direction.
Keywords: CBD, cannabidiol, hemp, novel food.
Cannabis is an important herbaceous plant species originating from Central Asia, which has been used in traditional herbal medicine and as a source of textile fibre for millennia (Andre et al., 2016). It is a fast-growing plant which has seen increased interest from the biotechnology, pharmaceutical and construction industries due to the potential multi-purpose applications of its phytochemicals and fibres.
Cannabis satvia L varieties all produce a unique family of natural plant compounds called cannabinoids. Cannabis plants are routinely categorized by their chemical phenotype or "chemotype", based on the overall amount of THC (tetrahydrocannabinol) produced, and on the ratio of THC to CBD. Colloquially, the species are categorised as “hemp” (low THC) or “marijuana” (high THC).
These different types of Cannabis plant varieties are cultivated for different purposes. Those varieties cultivated for hemp fibre and seed production typically have very low levels of THC, contain higher percentages of CBD and cause no psychoactive effects (described as low-intoxicant, non-drug, or fibre types). Those cultivated for drug production have lower percentages of CBD and levels of THC 5% - 35% w/w as the principal psychoactive ingredient in flowers, buds and leaves (described as high-intoxicant or drug types). These are the varieties used for recreational drugs. There can also be escaped, hybridised, or wild forms of either of the above types.
Cannabis Plant Cultivation
In the EU / UK cannabis is a controlled drug - unlawful to possess, supply, produce, import or export this drug or to cultivate any plant of the genus Cannabis except under a licence. The cultivation of Cannabis sativa L. varieties is only permitted in the EU and UK to produce hemp products when the variety is registered in EU’s ‘Common Catalogue of Varieties of Agricultural Plant Species’ and when THC content ≤0.2 %w/w (EU Regulation 1307/2013).
Similarly, in the USA, but with notable differences in permitted THC levels, cultivation of Cannabis sativa L. varieties are permitted as federally legalized hemp where levels of THC are <0.3% w/w (FDA, 2020).
What is Hemp?
Hemp is a common name for the low-intoxicant Cannabis plant in English and for its seeds, leaves, roots which are widely used in food, food supplements, rope, clothing, paper, board, soaps, and other cosmetics.
Hemp seeds, hemp seed oil, hemp seed flour, and defatted hemp seed all have a history of consumption in the EU and are therefore not considered novel foods. They, therefore, do not require authorisation to be used in foods providing they have been produced from registered varieties grown under licence.
Hemp and hemp-derived products are permitted also in the USA when derived from low THC content hemp varieties, grown under licence. There are GRAS (generally regarded as safe) notices accepted and in place (FDA, 2018) for:
- Hemp seed oil and hemp seed protein for use as an ingredient and food additive for general use in foods, excluding USDA/FSIS regulated products and infant formula
- De-hulled hemp seed for use as an ingredient in a variety of beverages, baked goods, cereals, spreads and snacks
What are Cannabinoids?
Cannabinoids are a class of compounds that, along with other terpenoids and flavonoids, are found in cannabis plants. Over 100 such natural phyto-cannabinoids have been identified. The major ones have been characterised in terms of their toxicological properties, but some minor compounds are less well characterised. The mix of cannabinoids in any plant extract is highly dependent upon the cannabis species and which part of the plant.
The most common natural cannabinoids include delta-9-tetrahydrocannabinol (Δ9-THC), and its precursor in marijuana, delta-9-tetrahydrocannabinolic acid A (Δ9-THCA-A) (Note: THC is psychoactive but THC-A has no psychotropic effect as long as it is not heated), delta-9-tetrahydrocannabinolic acid B (Δ9-THCA-B), delta-8-tetrahydrocannabinol (Δ8-THC), CBD, and its precursor in hemp cannabidiolic acid (CBDA), cannabigerol (CBG), cannabinol (CBN), cannabichromene (CBC) and delta-9-tetrahydrocannabivarin (Δ9-THCV) (Andre et al, 2016; Lafaye et al 2017). The most widely researched cannabinoids are ∆9-THC and CBD. THC is the primary constituent of cannabis that causes the “high” whereas CBD is not intoxicating at typical doses. (Freeman et al, 2019)
Cannabidiol is one of the main cannabinoid components, particularly in extracts from non-psychoactive cannabis species. The World Health Organisation considers purified CBD to be generally well tolerated with a good safety profile for use in medicines (WHO, 2018). The potential medical applications of CBD have been investigated and researched, including its use in clinical trials for the treatment of epilepsy and seizures. There is also preliminary evidence that cannabinoids may be useful therapeutic compounds for several other medical conditions such as nausea, chronic pain, and other neurological conditions such as multiple sclerosis (Freeman et al, 2019). Adverse effects may occur as a result of drug-drug interactions between CBD and patients’ existing medications. CBD containing products taken for the purpose of prevention or treatment of disease should be treated as medicines and need to be formally approved and licensed as such. There are now approved cannabinoid medicinal products in the EU: Marinol and Syndros (active ingredient dronabinol), Cesamet and Canemes (active ingredient nabilone) and Sativex (active ingredient nabiximols), and in the USA: Epidiolex (active ingredient CBD) (EMCDDA, 2018a).
Non-medicinal CBD-containing products are becoming increasingly popular and have now entered both the food and cosmetics sector. There are several CBD products on the market in the cosmetic sector for topical use. These include but are not limited to serums, creams, washes/rinse-off products (cleansers, shampoos, conditioners, body washes, masks), bath products (capsules, oils, tablets and salts), deodorants, balms and toothpastes. CBD-containing cosmetic products do not require pre-market authorisation. These products may contribute to CBD exposure via dermal absorption.
CBD can be found in a wide range of food products, including beverages (beer, spirits, wine, coffee and soda style drinks), oils (tinctures, drops, syrup, olive oils), chewables (gum drops, chewing gum) and chocolate. The regulation and legality of such products are complex and highly dependent on the jurisdiction in which they are sold. They are legal in the many US States, but in the European Union, there are a number of regulatory hurdles to their legal sale, summarised in Figure 1.
Figure 1. Legal Sale of Cannabis-Derived Ingredients in the EU
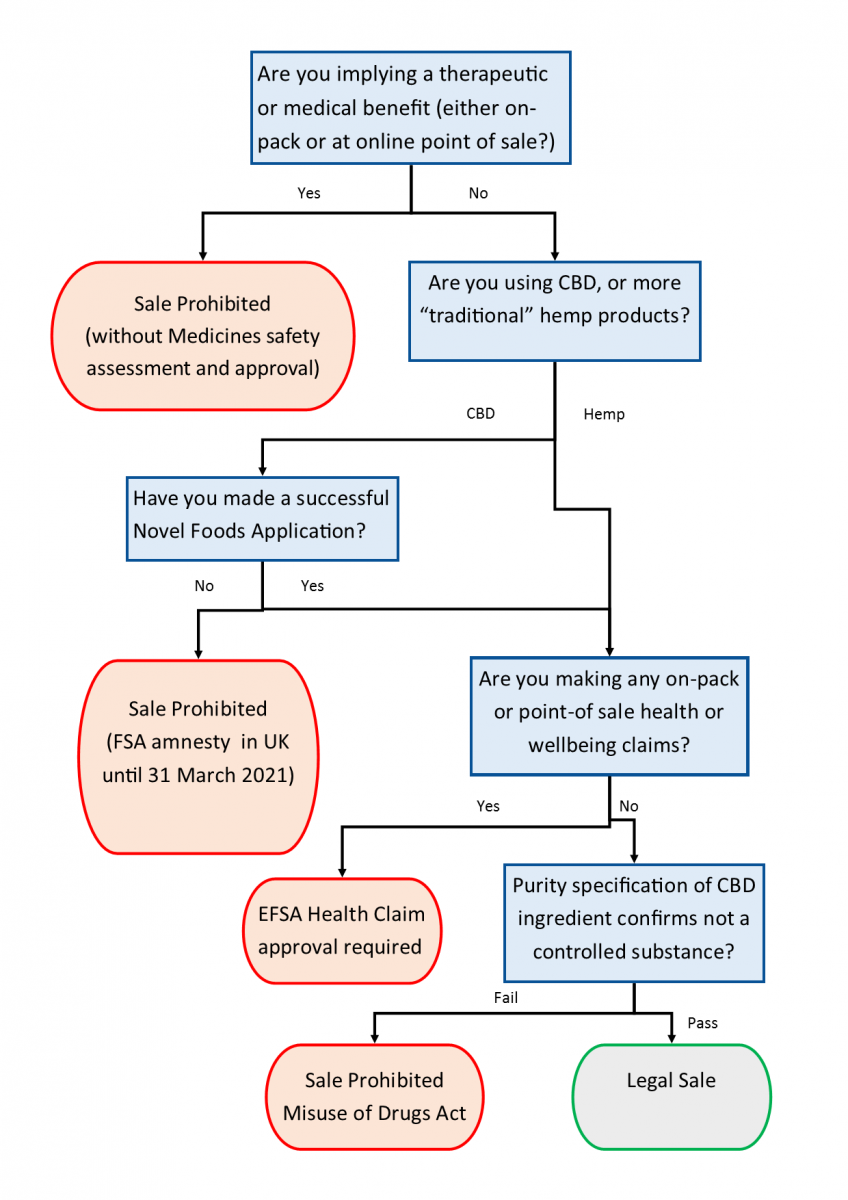
In the EU, any product which self-defines as a medicine by making a therapeutic claim cannot be sold without a Marketing Authorisation (MA). An MA is only granted after submission and approval of a registration study that demonstrates medicinal efficacy and safety. In the UK, this is administered by the Medicines and Healthcare Regulatory Agency MHRA.
In the USA the legal position is less clear. The Food and Drug Administration (FDA) considers CBD from any source as an active pharmaceutical ingredient requiring drug authorisation. Both THC and CBD, the prominent cannabinoids found in cannabis, have both been utilized in the development of FDA-approved drugs, e.g. FDA approved Epidiolex (0.1% THC) in June 2018. It is illegal in the USA to introduce or deliver into interstate commerce any food (including animal food or feed) with an added substance with an active drug ingredient. Ingredients first investigated/approved as a drug cannot be dietary supplements/dietary supplement ingredients (DSHEA, 1994 and 21 USC Section 201 (ff)(3) (a)-(B); FDA, 2020). Despite interstate commerce being illegal under Federal Law, individual States have considerable legal autonomy, and most States allow the use of both CBD and hemp in food and supplements.
Extracts of Cannabis sativa L. and derived products containing cannabinoids (and any products to which they are added) were confirmed in the EU/UK as novel foods in January 2019 as there is no significant history of consumption in the EU and they need to be authorised before being placed on the market. At the time of this legal clarification, many CBD-containing foods were already on the market. The approach to enforcement with respect to these products varies amongst EU Member States. The FSA is giving the CBD industry a deadline of 31 March 2021 to submit valid novel food authorisation applications. After 31 March 2021, only products which have submitted a valid application will be allowed to remain on the market. In other countries, products have been withdrawn or barred from sale.
Novel foods approval applies to each CBD production process and each cannabis species. It is therefore unlikely that a single application can cover the whole CBD industry. Each CBD producer needs an approved application that is specific to their own product. Synthetically obtained cannabinoids are also considered as novel foods/food ingredients.
Where CBD extracts also contain THC (or other controlled cannabinoids) then they will likely fall under the Misuse of Drugs Act 1971, and further guidance is available from the Home Office through a factsheet on Cannabis, CBD and other cannabinoids (UK Home Office, 2019). THC has been detected as an impurity in many CBD products and has led to a number of recalls in both the US and Europe. There is currently no lower legal limit so an appropriate approach would be to apply a zero limit for THC until enforcement authorities have adopted formal limits of acceptance for adventitious THC contamination.
The UK FSA is advising those who are pregnant, breastfeeding or taking any medication not to consume CBD products. Healthy adults are also advised to think carefully before taking CBD, and the FSA recommends no more than 70mg a day (about 28 drops of 5% CBD) unless under medical direction. This new precautionary advice is based on findings by the government’s Committee on Toxicity (COT) (COT, 2020). The COT is an independent scientific committee that provides advice to the UK FSA, Department of Health and Health Care and other Government Departments and Agencies on matters concerning the toxicity of chemicals.
Since 2015, the European CBD industry has grown exponentially. Many of the new businesses had no history in food production. It is important to assure that any CBD supplier has the safety infrastructure and systems in place that are expected within a food business. This would be expected to include good agriculture practices for primary agriculture, good manufacturing practices and HACCP (hazard analysis critical control points) for food and food supplements manufacturing and pharmaceutical good manufacturing practices for medicines.
Given the complex and differing regulatory frameworks around CBD, it is recommended to seek expert advice as to the regulatory requirements for the intended market of sale relating to CBD in food and food supplement products. Any disease treatment or prevention of disease claims should not be made as this is not permitted for foods, only for licensed medicines. Companies considering using CBD extracts in products should ensure they have detailed ingredient specifications with confirmatory batch analytical test results to ensure the CBD extract would not be considered a controlled substance. Best practice food safety assurance should also be applied to ensure control of contaminants such as heavy metals, adulterants typical of produce/herbs, and bacterial and viral pathogens. CBD products should be clearly labelled with direction for use appropriate for the intended use and product category type. Warnings should be clearly stated on pack appropriate for intended use and which include FSA recommendations i.e. ‘Keep away from children’, ‘Not suitable for those who are breast-feeding, pregnant or taking any medication’.
Andre, C. M., Hausman, J-F., and Guerriero, G. (2016) Cannabis sativa: The Plant of the Thousand and One Molecules Front Plant Sci. 7: 19. doi: 10.3389/fpls.2016.00019
Committee On Toxicity Of Chemicals In Food, Consumer Products And The Environment (COT) (2020) CBD Update TOX/2020/02 https://cot.food.gov.uk/sites/default/files/tox202002cbd.pdf
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2018a) Medical use of cannabis and cannabinoids. ISBN 978-92-9497-362-7 doi:10.2810/979004
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2018b) Cannabis legislation in Europe. An overview. doi:10.2810/566650 ISBN 978-92-9497-328-3
Freeman, T.P, Hindocha, C., Green, S.F., Bloomfield, M.A.P. (2019) BMJ 2019;365:l1141 doi: 10.1136/bmj.l1141
Food and Drug Administration (FDA) (2018) FDA Responds to Three GRAS Notices for Hemp Seed-Derived Ingredients for Use in Human Food 20 December 2018. Accessed 13 February 2020. https://www.fda.gov/food/cfsan-constituent-updates/fda-responds-three-gras-notices-hemp-seed-derived-ingredients-use-human-food
Food and Drug Administration (FDA) (2020) FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD) 15 January 2020. Accessed 13 February 2020. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd
Lafaye, G., Karila, L., Blecha, L., and Benyamina, A. (2017) Cannabis, cannabinoids, and health. Dialogues Clin Neurosci. 2017 Sep; 19(3): 309–316.
Levinsohn E.A. and Hill K.P. (2020) Clinical uses of cannabis and cannabinoids in the United States. J Neurol Sci. 411:116717. doi: 10.1016/j.jns.2020.116717
European Commission (2013) Commission Regulation (EU) No 1307/2013 of the European Parliament and of the Council of 17 December 2013 establishing rules for direct payments to farmers under support schemes within the framework of the common agricultural policy and repealing Council Regulation (EC) No 637/2008 and Council Regulation (EC) No 73/2009 OJ L 347, 20.12.2013, p. 608–670 https://eur-lex.europa.eu/eli/reg/2013/1307/oj
World Health Organisation (WHO) (2018) CANNABIDIOL (CBD) Critical Review Report World Health Organisation Expert Committee on Drug Dependence Fortieth Meeting Geneva, 4-7 June 2018 https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf
UK Home Office (2019) Guidance - Cannabis, CBD and other cannabinoids: drug licensing factsheet v. 1.4 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/825872/factsheet-cannabis-cbd-and-cannabinoids-2019.pdf
Institute of Food Science & Technology has authorised the publication of the following (new) Information Statement on Cannabidiol (CBD) and Cannabinoids, dated October 2020.
This Information Statement has been prepared by Rachel Ward, FIFST, peer-reviewed by professional members of IFST and approved by the IFST Scientific Committee.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
